Recent Publications
| 94. | Interaction Force Landscapes within and Surrounding Hydrophobic Nanopockets Revealed by Three-Dimensional Scanning Atomic Force Microscopy M. Ogasawara, S. Tanaka, K. Komatsu, N. Kishimoto, D. Zhang, T. Yoneda, Y. Inokuma, M. Morimoto, A. Ohta, H. Asakawa J. Phys. Chem. C 2026, 130, 1004-1013. 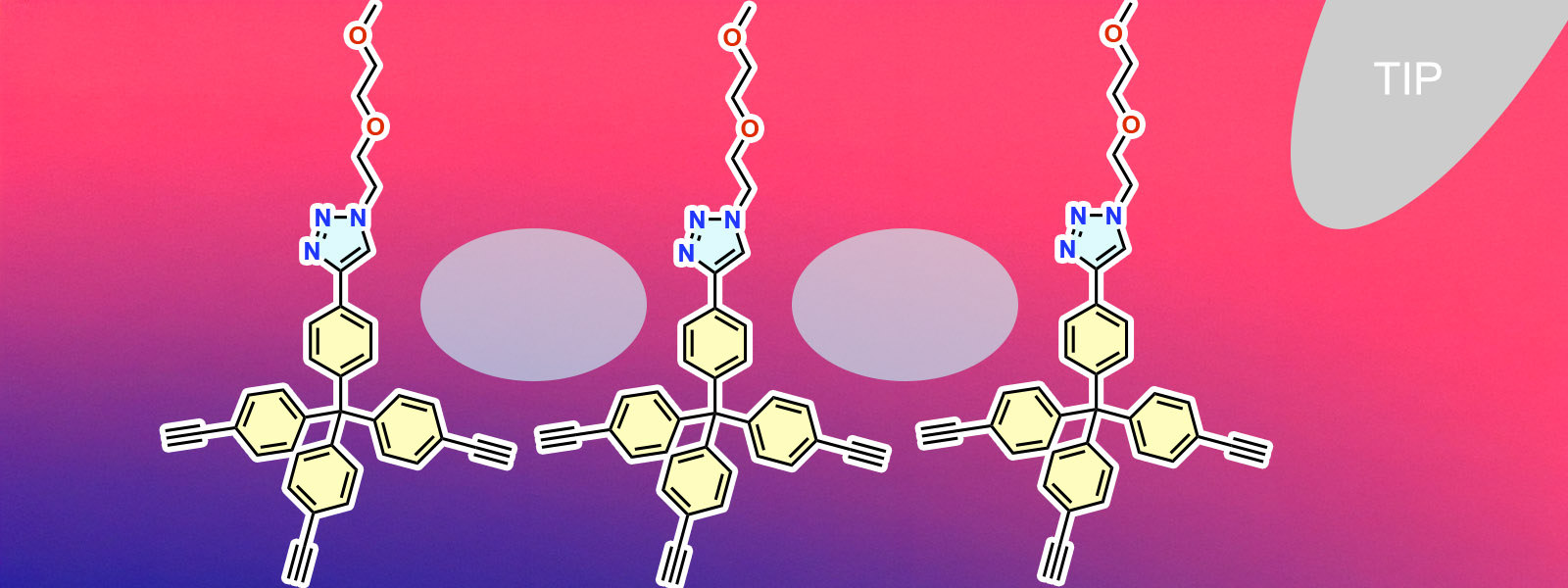 Collaboration with Prof. Asakawa and Kishimoto. |
| 93. | Click Conjugation Using Discrete Polyketones Enables Dual Anchoring of Antibodies and Nanowires for Exosome Profiling K. Chattrairat, A. Yokoi, Y. Manabe, Y. Ide, T. Hasegawa, M. Iida, T. Ajiri, Z. Zhu, R. Uekusa, M. Kitagawa, Y. Baba, H. Kajiyama, Y. Inokuma, T. Yasui ChemRxiv DOI: 10.26434/chemrxiv-2025-n859x 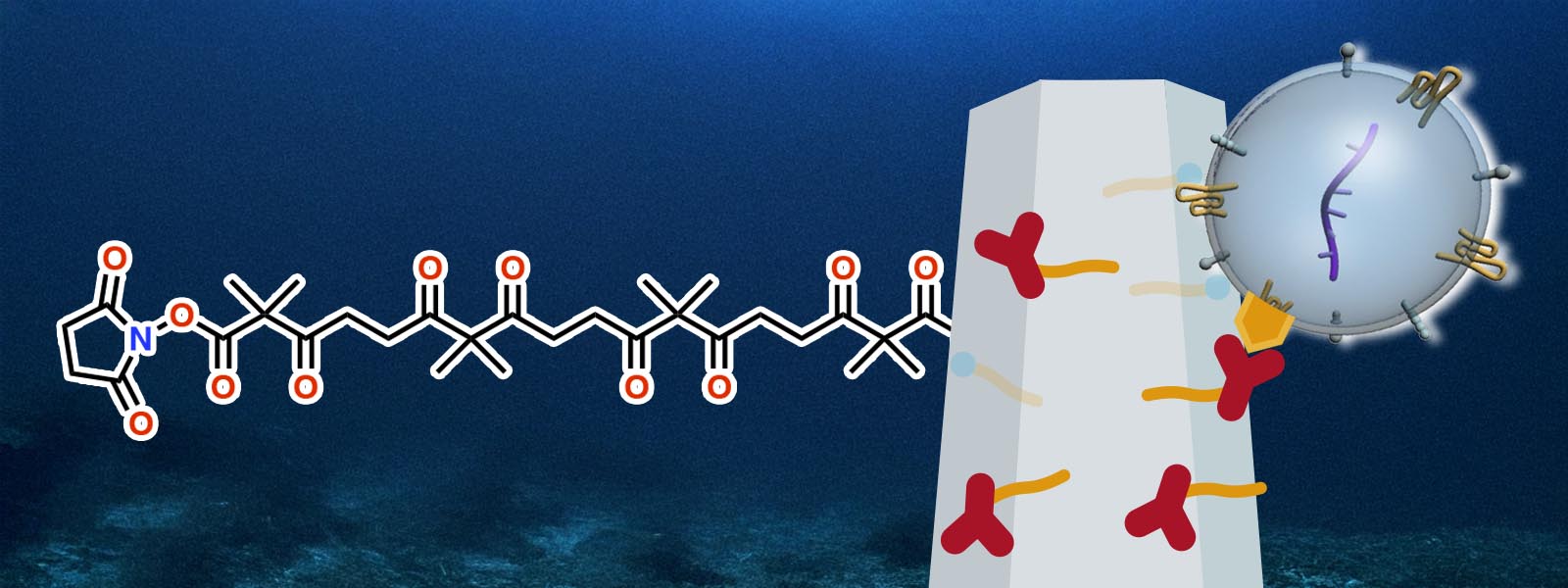 A facile bioconjugation protocol that connects ZnO nanowires with antibodies was developed using terminally functionalized polyketones. Collaboration with Prof. Yasui at Science Tokyo. |
| 92. | Image-Based Machine Learning Using Inkjet-Printed Chemicals: Mixing Ratio Prediction and Metal Ion Detection T. Sano, Y. Terauchi, Y. Ide, I. Takigawa, T. Minami, Y. Inokuma Org. Lett. 2025, 27, 8841-8845. 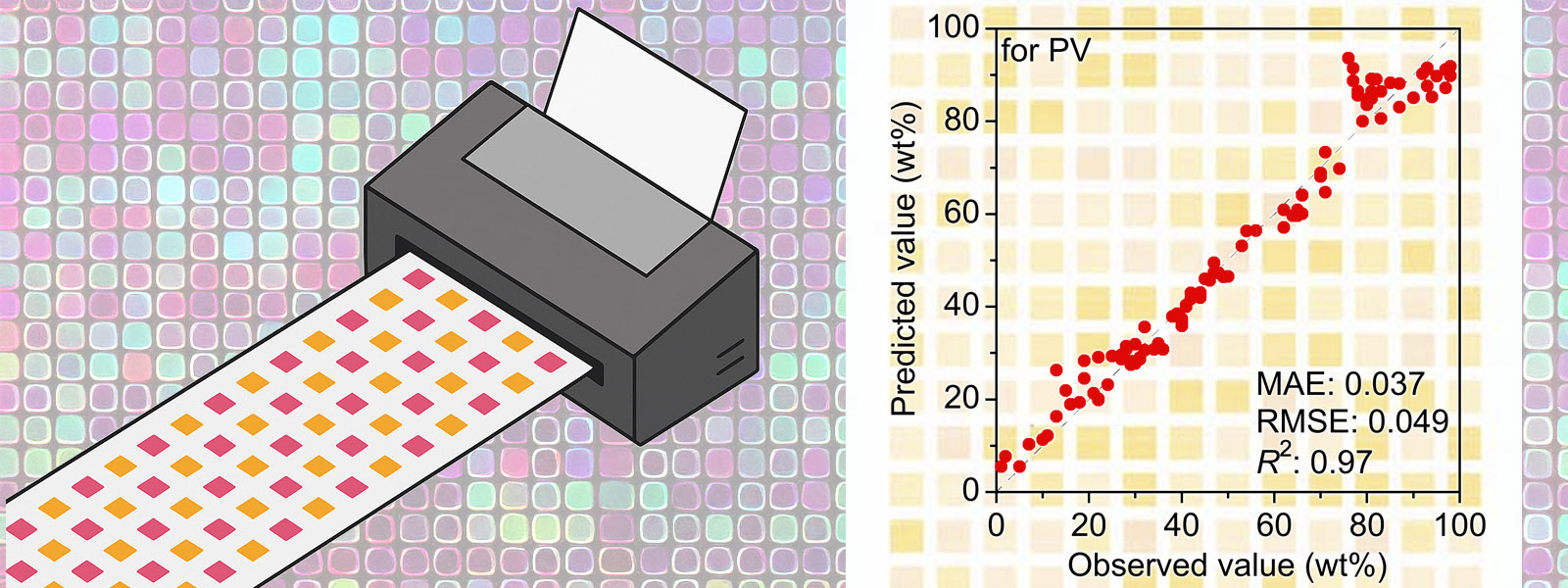 A quick and cost-efficient protocol using inkjet printing to collect training datasets for image-based machine learning. This work is a collaboration with Prof. Takigawa (ICReDD) and Prof. Minami (U. Tokyo). Also selected for the front cover! |
| 91. | Size-Selective Synthesis of Calix[n]furan[2n]thiazoles Bearing meso-CH2 Bridges K. Shibata, T. Nakabayashi, K. Watanabe, Y. Ide, T. Ichino, Y. Inokuma J. Porphyrins Phthalocyanines 2025, 29, 184–190. 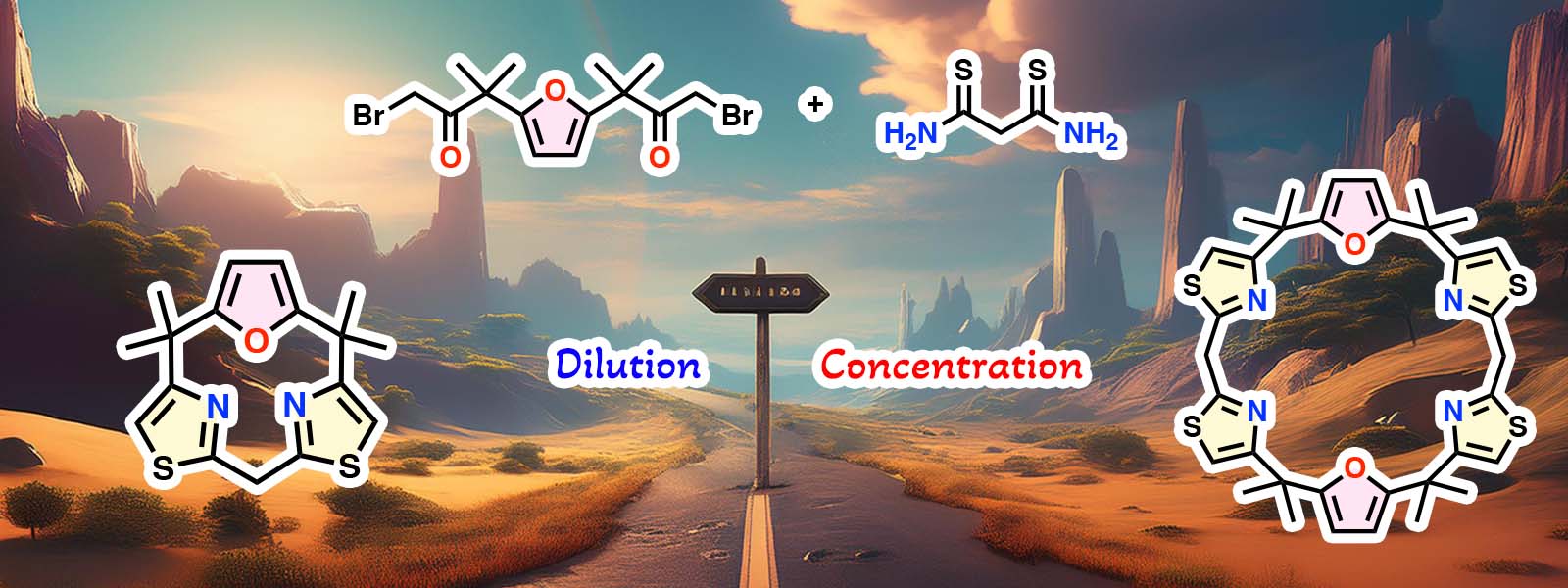 Invited Article dedicated to Prof. Karl M. Kadish on the occasion of his 80th birthday. |
| 90. | Cyclo[4]pyrrole with α–β Direct Linkages Y. Sun, R. Kitahara, T. Ichino, Y. Ide, H. Senboku, S. Shimizu, T. Tanaka, Y. Inokuma Chem. Sci. 2024, 15, 19571–19576. 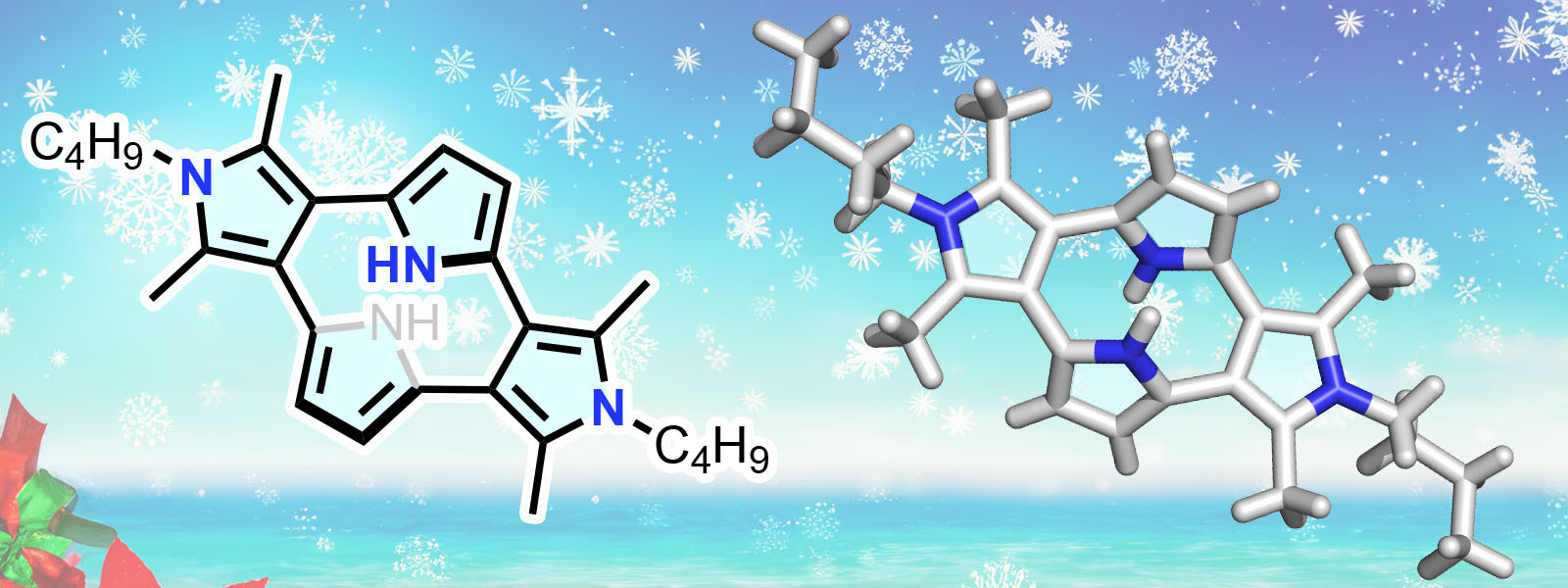 A joint research with Prof. Shimizu (Kyushu Univ.), Prof. Tanaka (Kyoto Univ.) and Dr. Ichino (ICReDD). |
| 89. | Hydride Content Control of Perovskite Oxyhydride BaTiO3–xHx Supported by Image-Based Machine Learning T. Sano, Y. Ide, T. Tsumori, H. Ubukata, I. Takigawa, H. Kageyama, Y. Inokuma ACS Appl. Eng. Mat. 2024, 2, 2391–2396.  Image-based machine learning prediction system used to accurately and non-destructively determine hydride content in perovskite oxyhydride. |
| 88. | A Molecular Dynamics-Based Conformational Simulation Method for Analysis of Arrival Time Distributions in Ion Mobility Mass Spectrometry K. Tashiro, Y. Ide, T. Taketsugu, K. Ohara, K. Yamaguchi, M. Kobayashi, Y. Inokuma Adv. Theory Simul. 2024, 7, 202400691. 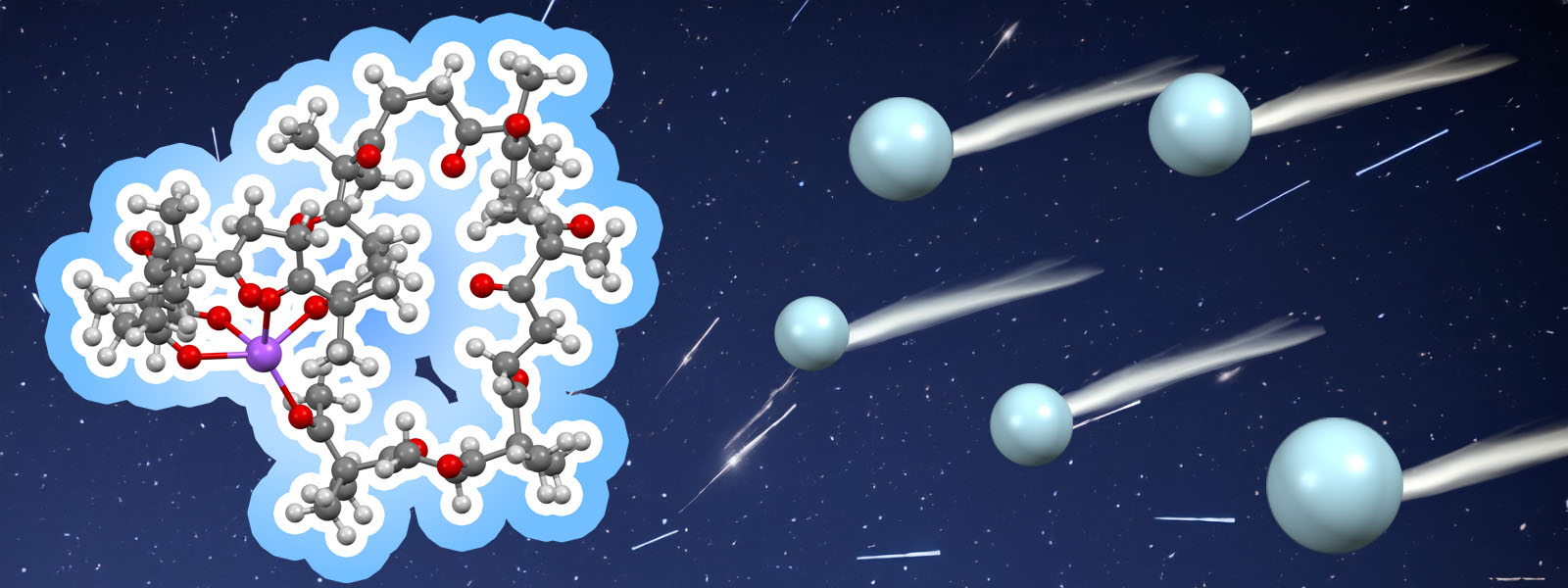 Collaboration with Profs. Kobayashi, Taketsugu, Ohara and Yamaguchi on CCS simulation of ion mobility mass spectrometry. |
| 87. | Discrete Polyketones: Synthesis, Derivatization, and Potential Applications Y. Inokuma Bull. Chem. Soc. Jpn. 2024, 97, uoae072. 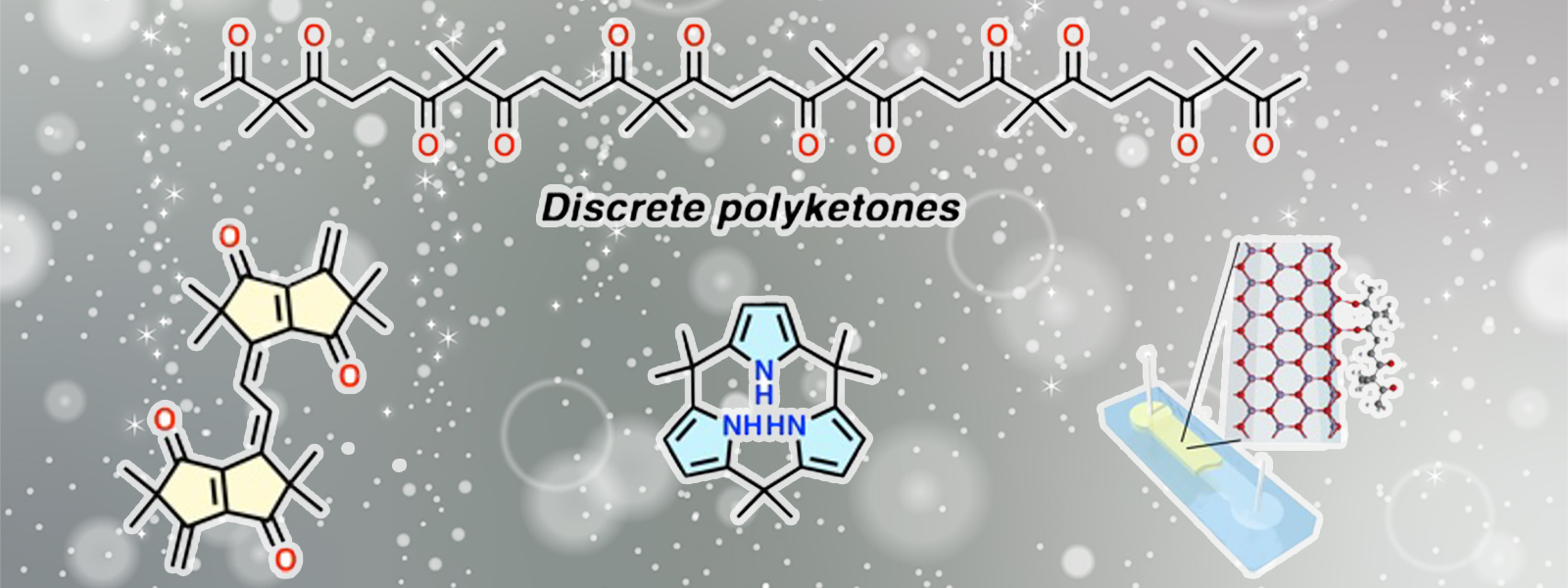 The award account for the Chemical Society of Japan Award for Creative Work for 2024. |
| 86. | Contracted Porphyrins and Calixpyrroles: Synthetic Challenges and Ring-Contraction Effects K. Watanabe, N. N. Pati, Y. Inokuma Chem. Sci. 2024, 15, 6994–7009. 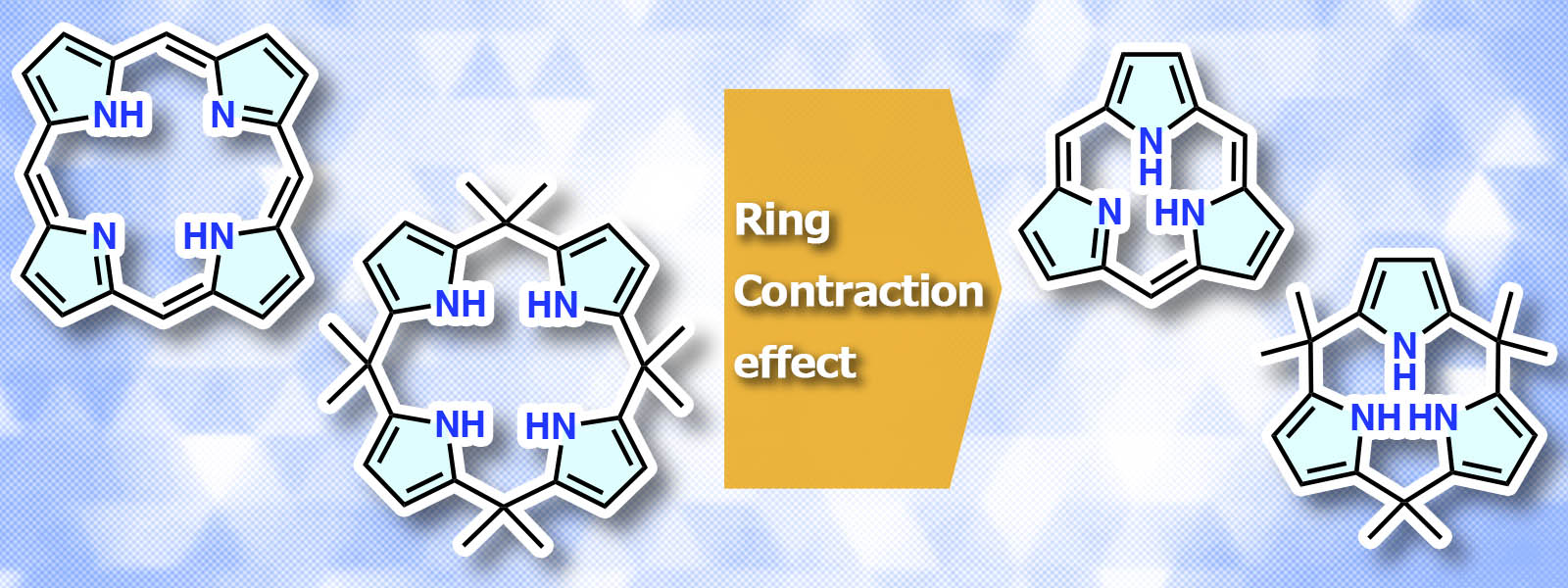 Unique ring-contraction effects are discussed for tripyrrolic porphyrin-related macrocycles. |
| 85. | Implementation of Highly Crystalline Polyketones as Solid Polymer Electrolytes in high-temperature Lithium Metal Batteries R. Andersson, I. L Johansson, K. I. Shivakumar, G. Hernández, Y. Inokuma, J. Mindemark Solid State Ionics 2024, 410, 116542. 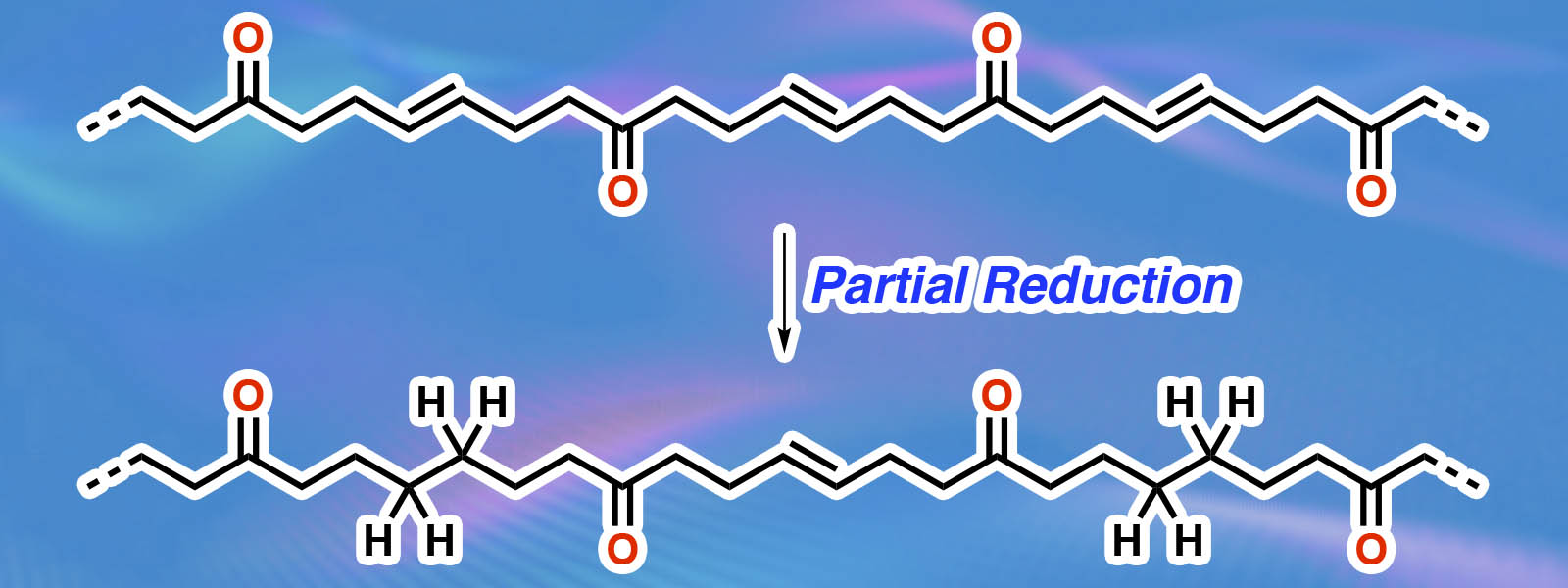 International collaborative research through the MANABIYA(Acedemic) program of ICReDD. |
| 84. | One-pot, Gram-Scale Synthesis of Calix[5]- and Calix[6]furan: Derivatization for Polyketone- and Isopyrazole-based Macrocycles with Conformational Analysis T. Yoneda, T. Sano, N. N. Pati, Y. Ide, Y. Inokuma Asian J. Org. Chem. 2024, 13, e202400023. 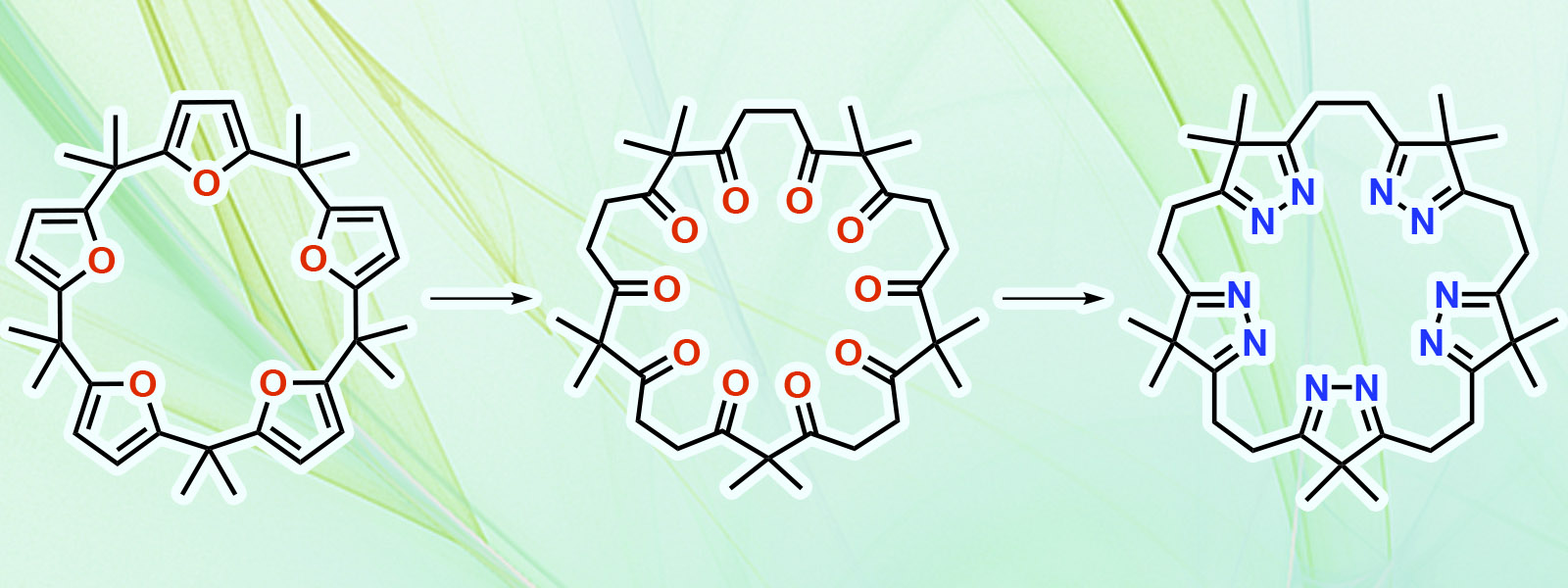 Practical gram-scale synthesis of calix[n]furans (n = 5, 6) and derivatization to polyketone and isopyrazole macrocycles |
| 83. | Strain-Based Design, Direct Macrocyclization, and Metal Complexation of Thiazole-Containing Calix[3]pyrrole Analogues K. Watanabe, K. Shibata, T. Ichino, Y. Ide, T. Yoneda, S. Maeda, Y. Inokuma Inorg. Chem. Front. 2024, 11, 3548–3554. ChemRxiv DOI: 10.26434/chemrxiv-2024-6nqnq 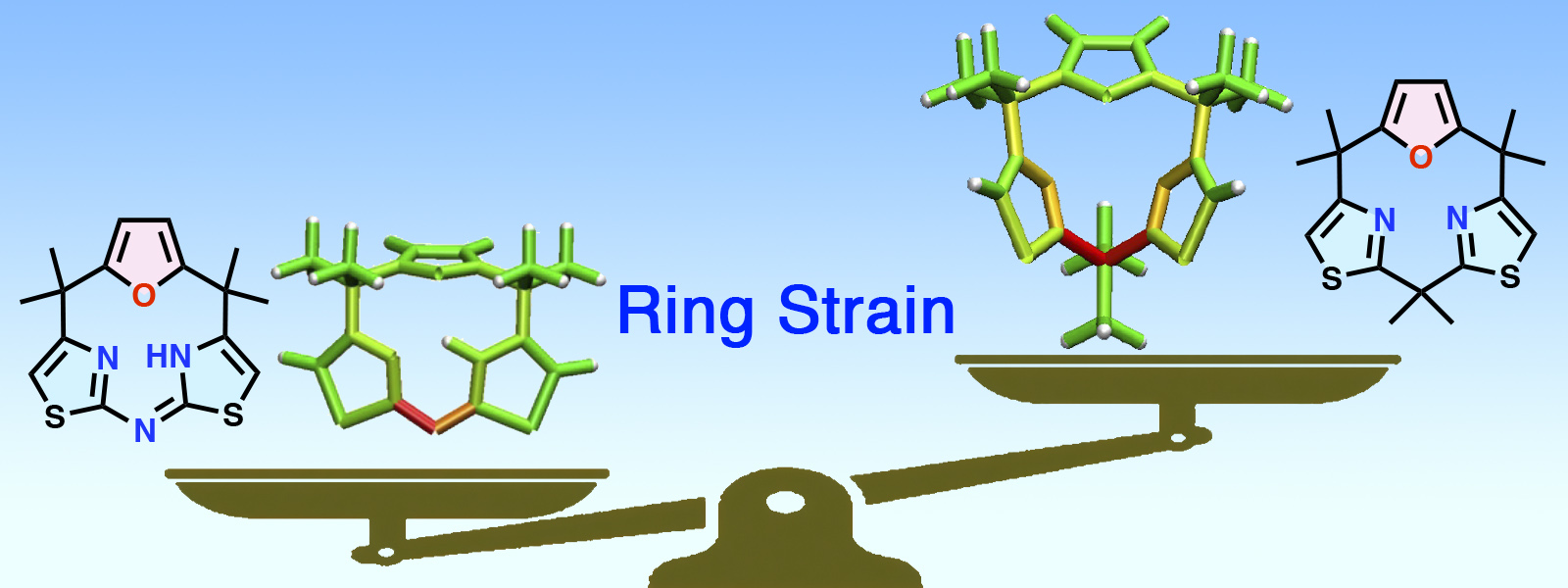 Evaluation and visualization of ring strain for molecular design of feasible calix[3]pyrrole analogues and their coordination chemistry in collaboration with Prof. Maeda's group |
| 82. | Drug–drug Conjugates of MEK and Akt Inhibitors for RAS-mutant Cancers H. Fujita, S. Arai, H. Arakawa, K. Hamamoto, T. Kato, T. Arai, N. Nitta, K. Hotta, N. Hosokawa, T. Ohbayashi, C. Takahashi, Y. Inokuma, I. Tamai, S. Yano, M. Kunishima, Y. Watanabe Bioorg. Med. Chem. 2024, 102, 117674. ChemRxiv DOI: 10.26434/chemrxiv-2023-ps4h1-v2 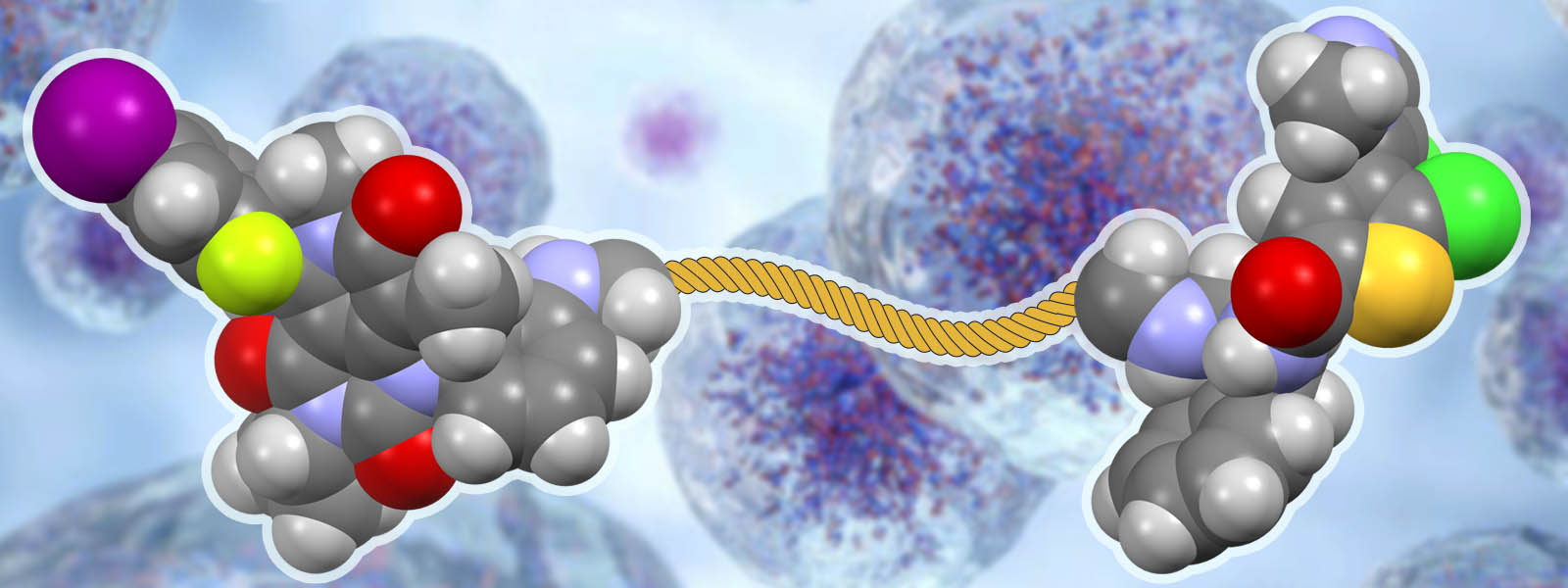 Collaboration research with medical researchers at Kanazawa University on cancer drug conjugates using carbonyl rope-related linkers |
| 81. | Machine Learning-Based Analysis of Molar and Enantiomeric Ratios and Reaction Yields Using Images of Solid Mixtures Y. Ide, H. Shirakura, T. Sano, M. Murugavel, Y. Inaba, S. Hu, I. Takigawa, Y. Inokuma Ind. Eng. Chem. Res. 2023, 62, 13790-13798. ChemRxiv DOI: 10.26434/chemrxiv-2023-3gdsb 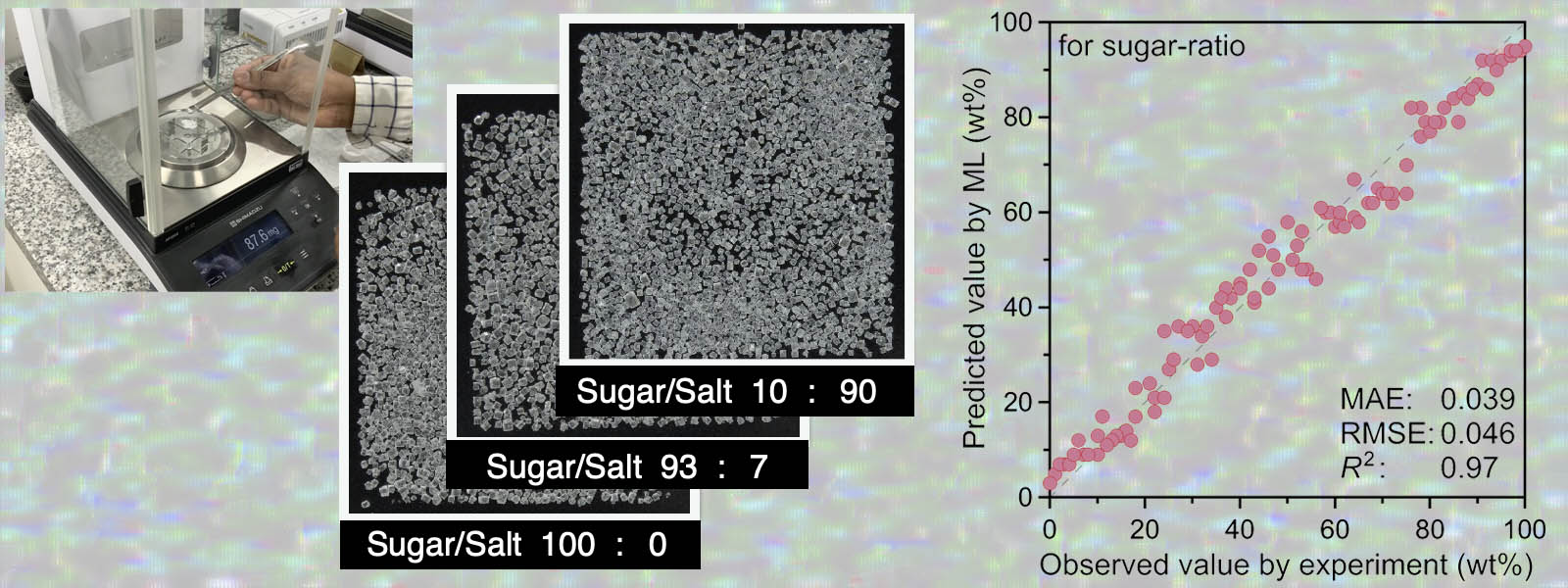 Image-based machine learning system capable of predicting mixing ratios of organic/inorganic compounds, polymorphs, and enantiomers (Selected as Cover Picture) Press release (Hokkaido University) |
| 80. | Identifying High-Grade Serous Ovarian Carcinoma-Specific Extracellular Vesicles by Polyketone-coated Nanowires A. Yokoi, M. Ukai, T. Yasui, Y. Inokuma, K. Hyeon-Deuk, J. Matsuzaki, K. Yoshida, M. Kitagawa, K. Chattrairat, M. Iida, T. Shimada, Y. Manabe, I-Y. Chang, E. Asano-Inami, Y. Koya, A. Nawa, K. Nakamura, T. Kiyono, T. Kato, A. Hirakawa, Y. Yoshioka, T. Ochiya, T. Hasegawa, Y. Baba, Y. Yamamoto, H. Kajiyama Sci. Adv. 2023,9, eade6958 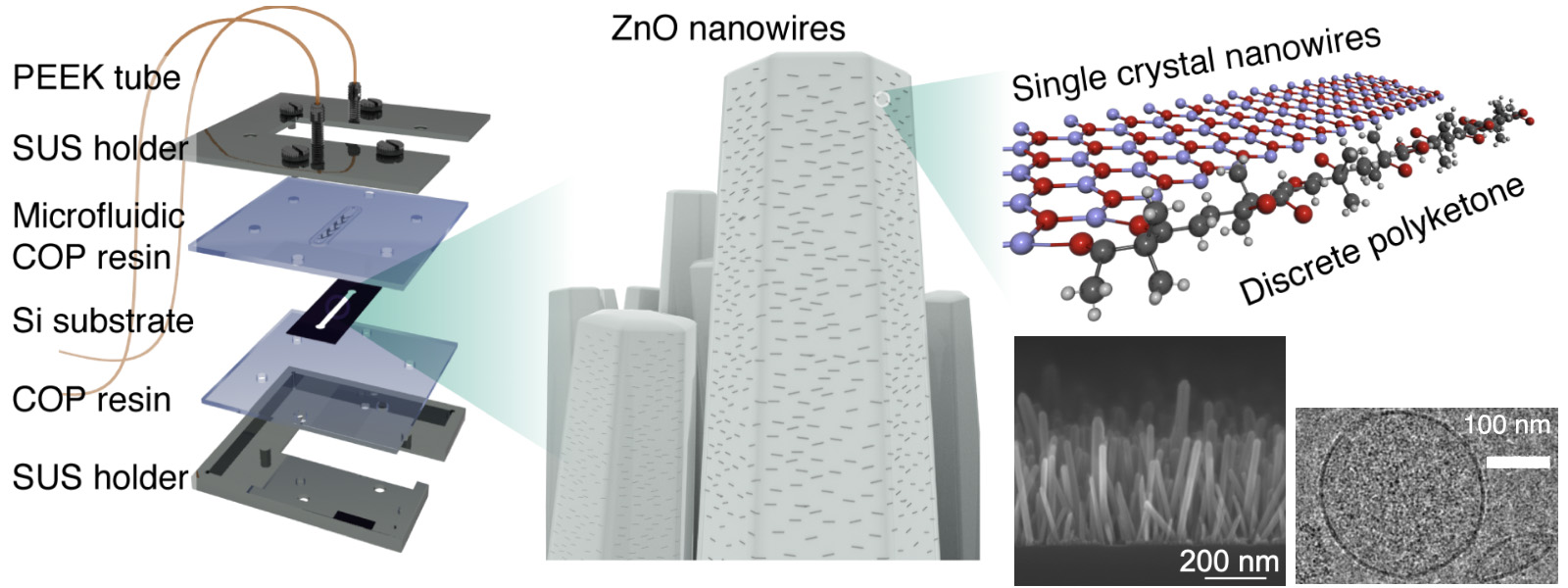 Device application of our polyketones for capturing high-grade serous ovarian carcinoma-specific extracellular vesicles |
| 79. | The Geometry of Calix[3]pyrrole and the Formation of the Calix[3]pyrrole·F–Complex in Solution R. Saha, J. Pirillo, Y. Ide, Y. Inokuma, Y. Hijikata Theor. Chem. Acc. 2023, 142, Article Number 50. 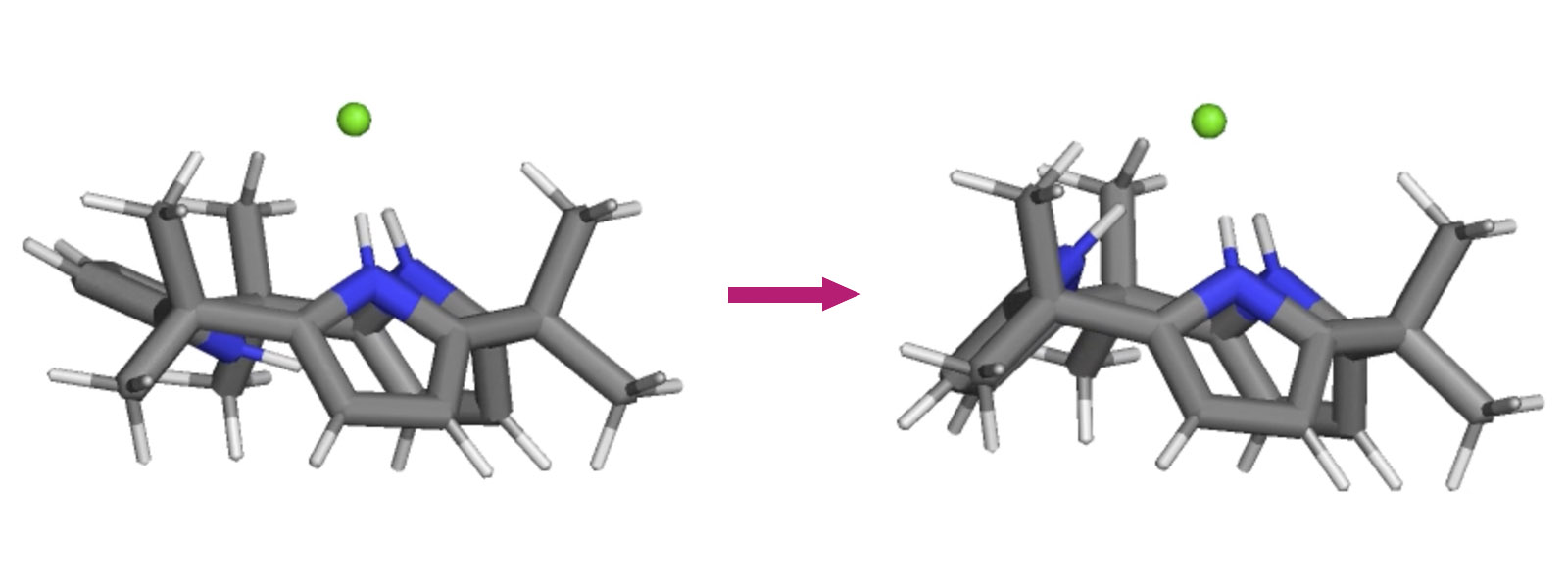 MD simulation study for calix[3]pyrrole–fluoride complexation with Hijikata group |
| 78. | Chain Length-Dependent Hydrogen-Bonded Self-Assembly of Terminally Functionalized Discrete Polyketones K. I. Shivakumar, Y. Manabe, T. Yoneda, Y. Ide, Y. Inokuma Precis. Chem. 2023, 1, 34-39. 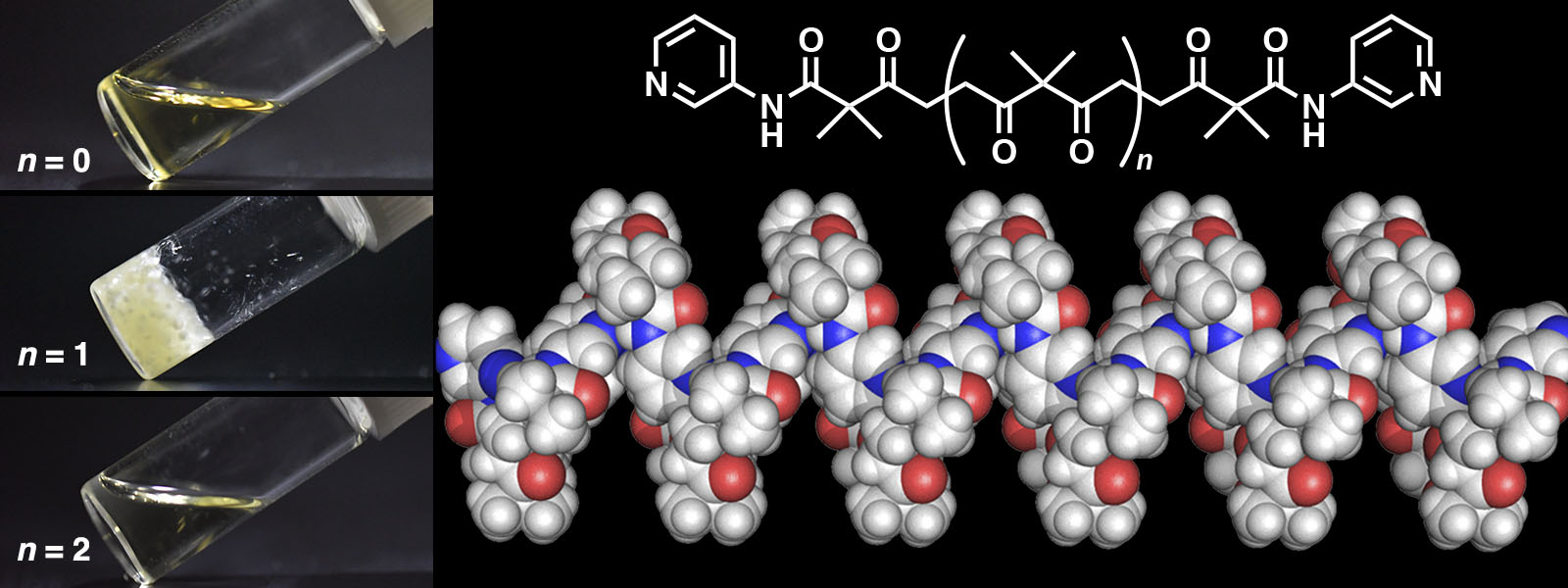 Hydrogen-bonding behaviors of terminally COOH or pyridine-substituted polyketones were analyzed. |
| 77. | Chiral Calix[3]pyrrole Derivatives: Synthesis, Racemization Kinetics, and Ring Expansion to Calix[9]- and Calix[12]pyrrole Analogues Y. Inaba, J. Yang, Y. Kakibayashi, T. Yoneda, Y. Ide, Y. Hijikata, J. Pirillo, R. Saha, J. L. Sessler, Y. Inokuma Angew. Chem. Int. Ed. 2023, 62, e202301460 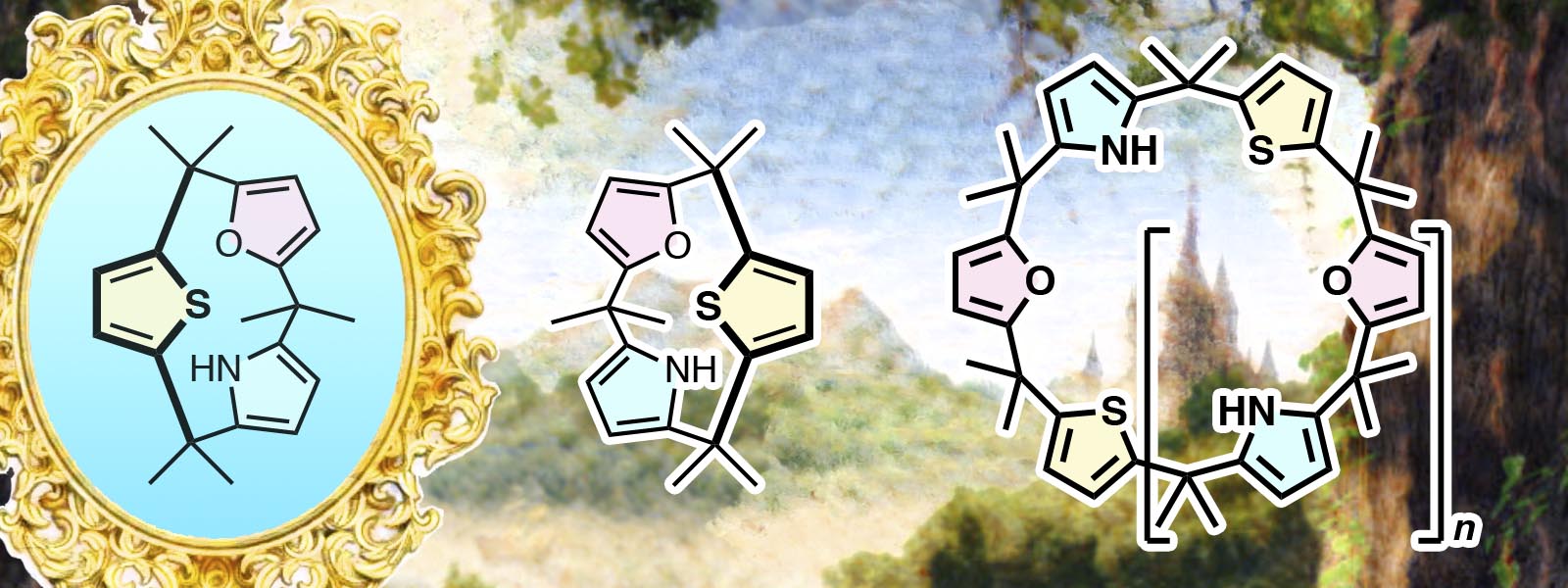 An inherently chiral calix[3]pyrrole derivative and its strain-induced ring expansion up to calix[12]pyrrole-type macrocycles |
| 76. | Toward Calix[2]-Type Macrocycles: Synthesis and Structural Analysis of Cyclic Tetraketone and Highly Strained Furanophane T. Sano, Y. Sun, T. Mukai, Y. Inaba, T. Yoneda, Y. Ide, J. Pirillo, Y. Hijikata, Y. Inokuma J. Porphyrins Phthalocyanines 2023, 27, 1067-1073. 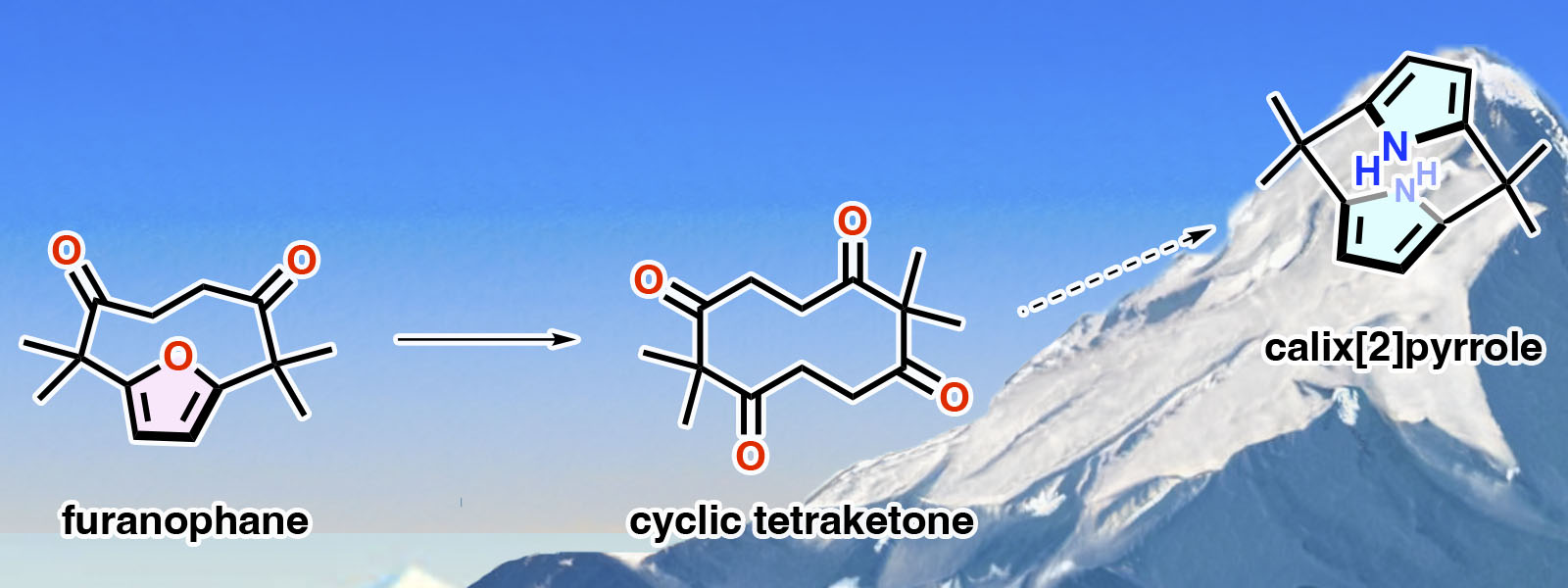 An odyssey for synthesis of calix[2]pyrrole started. Invited Article dedicated to Prof. Jonathan L. Sessler on the occasion of his 65th birthday. |
| 75. | Absorption Spectra of Calix[3]pyrrole Analogues as Probes for Contracted Macrocycles K. Watanabe, R. Saha, Y. Inaba, Y. Manabe, T. Yoneda, Y. Ide, Y. Hijikata, Y. Inokuma J. Porphyrins Phthalocyanines 2023, 27, 157-163. 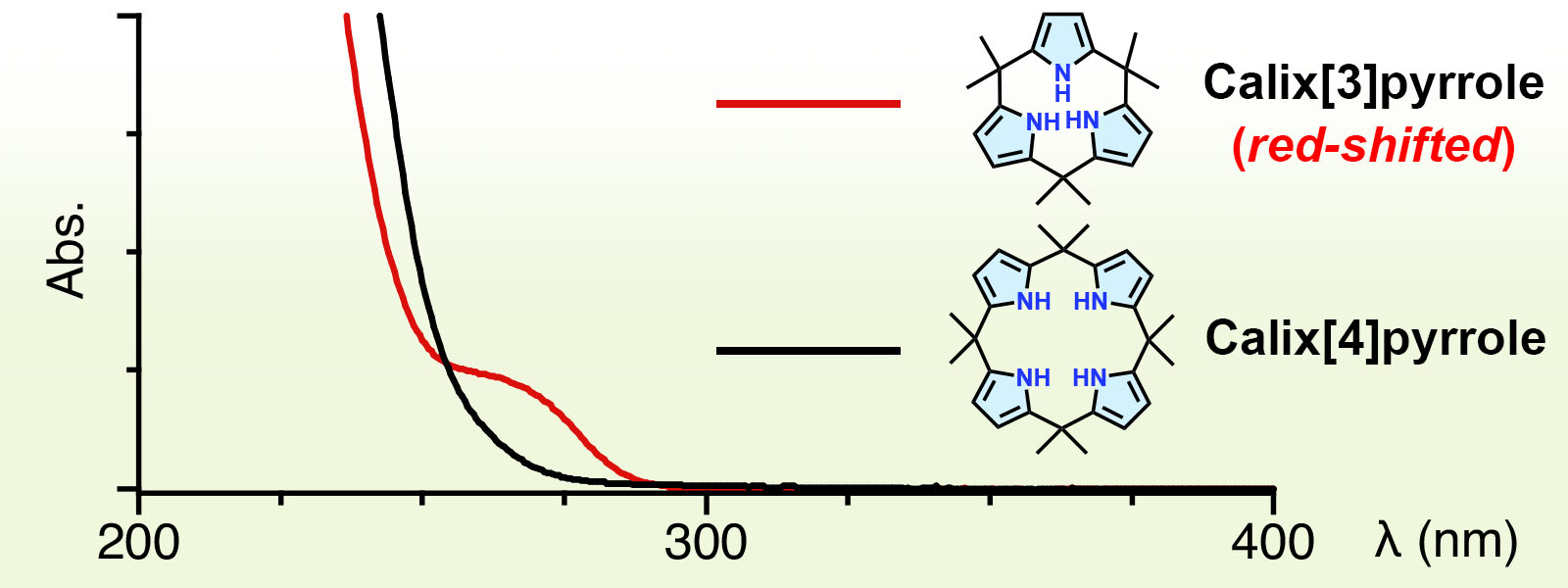 Characteristically red-shifted absorption band was observed for calix[3]pyrrole. Invited Article dedicated to Prof. Tomás Torres on the occasion of his 70th birthday. |
| 74. | Carbonyl-Containing Solid Polymer Electrolyte Host Materials: Conduction and Coordination in Polyketone, Polyester and Polycarbonate Systems T. Eriksson, H. Gudla, Y. Manabe, T. Yoneda, D. Friesen, C. Zhang, Y. Inokuma, D. Brandell, J. Mindemark Macromolecules 2022, 55, 10940-10949.  The 2nd collaboration paper with Prof. J. Mindemark group. |
| 73. | Determination of the Critical Chain Length for Macromolecular Crystallization Using Structurally Flexible Polyketones Y. Ide, Y. Manabe, Y. Inaba, Y. Kinoshita, J. Pirillo, Y. Hijikata, T. Yoneda, K. I. Shivakumar, S. Tanaka, H. Asakawa, Y. Inokuma Chem. Sci. 2022, 13, 9848–9854. 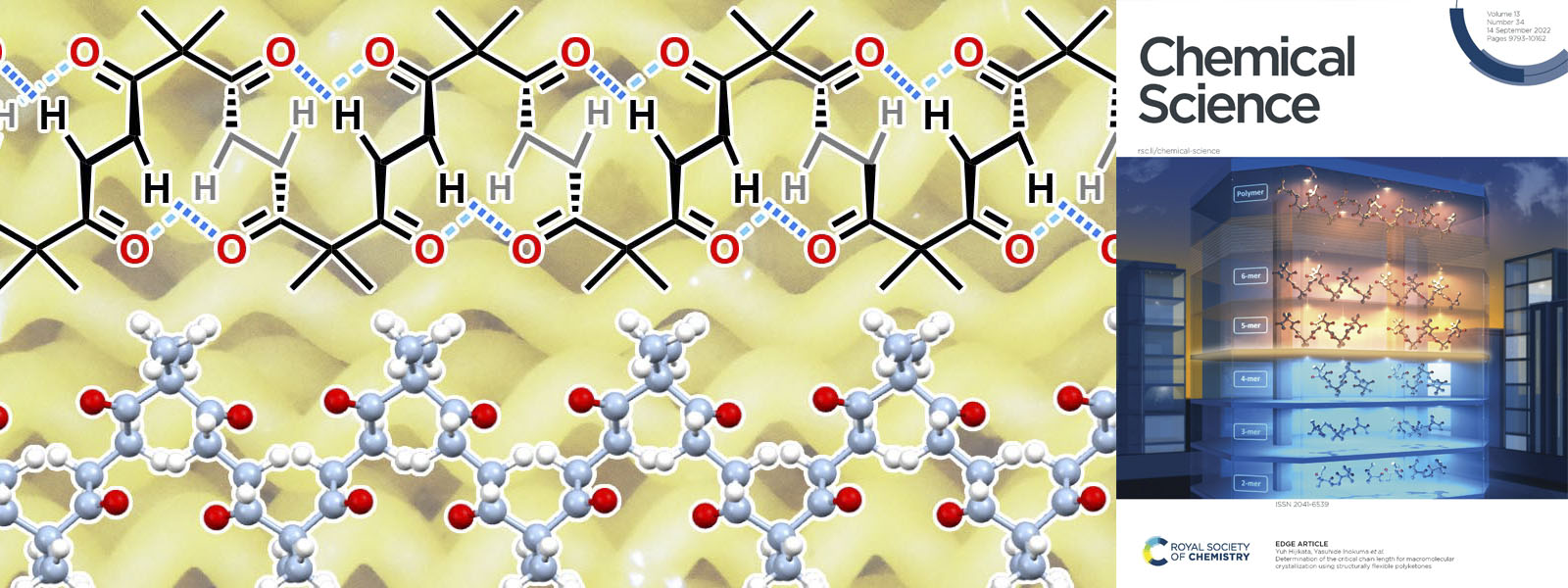 Critical chain length of macromolecular crystallization for carbonyl ropes was determied. Selected as Front Cover! |
| 72. | Alkali Metal Ion Binding Using Cyclic Polyketones N. Ozawa, K. I. Shivakumar, M. Murugavel, Y. Inaba, T. Yoneda, Y. Ide, J. Pirillo, Y. Hijikata, Y. Inokuma Chem. Commun. 2022, 58, 2971–2974. 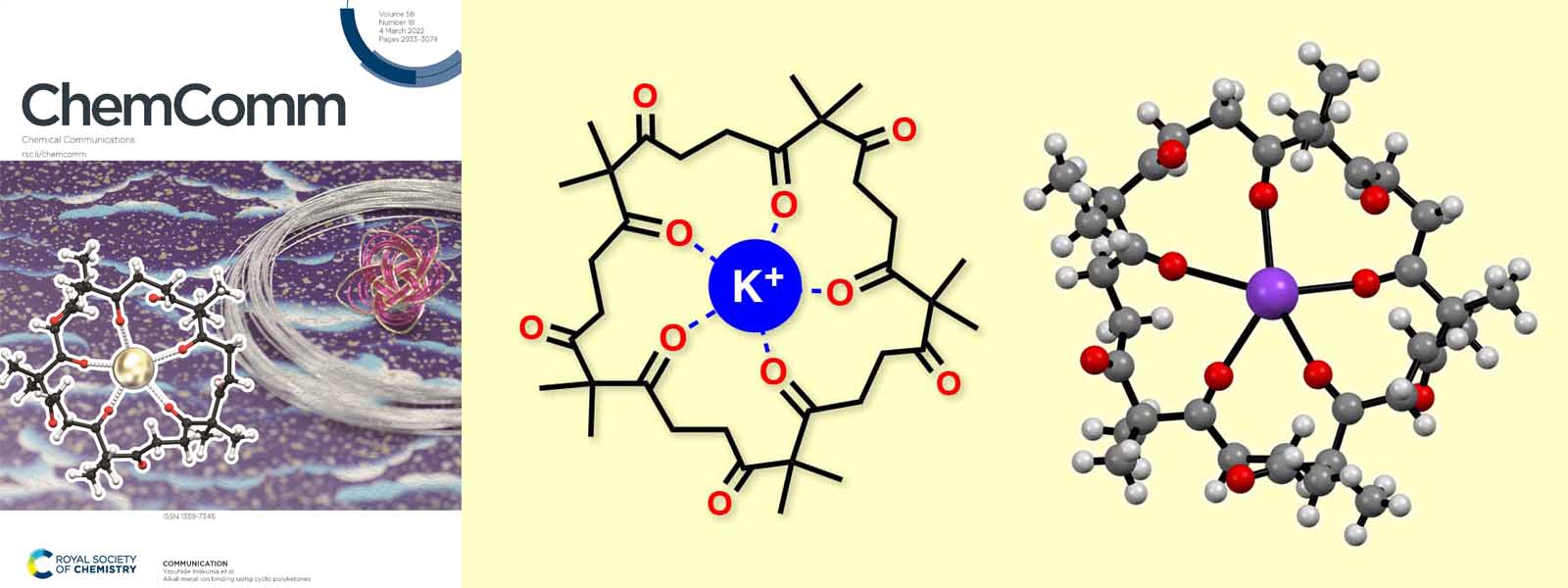 Alkali metal ion binding behavior of cyclic polyketones was discovered. (Selected as Inside Front Cover) Featured in Chemical Communications HOT Articles 2022. |
| 71. | Strain-induced Ring Expansion Reactions of Calix[3]pyrrole-related Macrocycles Y. Inaba, Y. Kakibayashi, Y. Ide, J. Pirillo, Y. Hijikata, T. Yoneda, Y. Inokuma Chem. Eur. J. 2022, 28, e202200056. 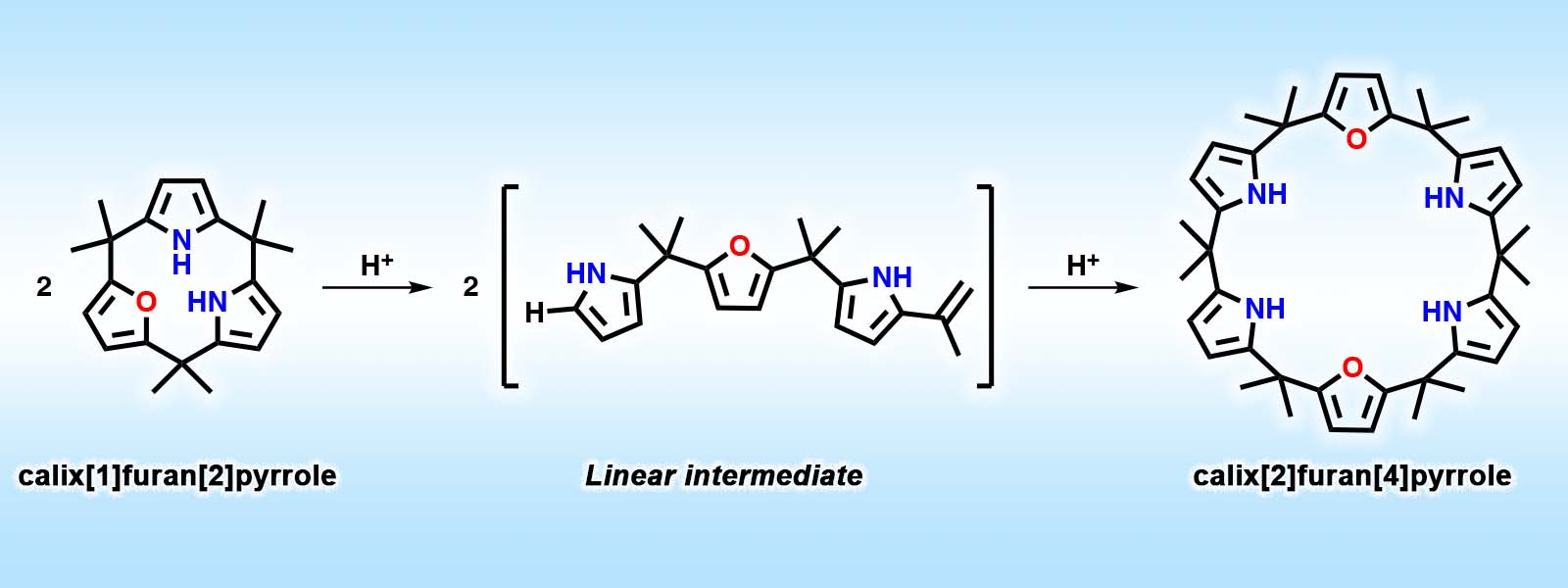 Analogues of calix[3]pyrrole were synthesized to investigate the scope and mechanism of ring expansion reaction. Chosen as "Hot paper" |
| 70. | Hybrid Eu(III) Coordination luminophore Standing on Silica Nanoparticles by Two Legs for Enhanced Luminescence T. Zhang, Y. Kitagawa, R. Moriake, P. P. F. Rosa, M. J. Islam, T. Yoneda, Y. Inokuma, K. Fushimi, Y. Hasegawa Chem. Eur. J. 2021, 27, 14438–14443. 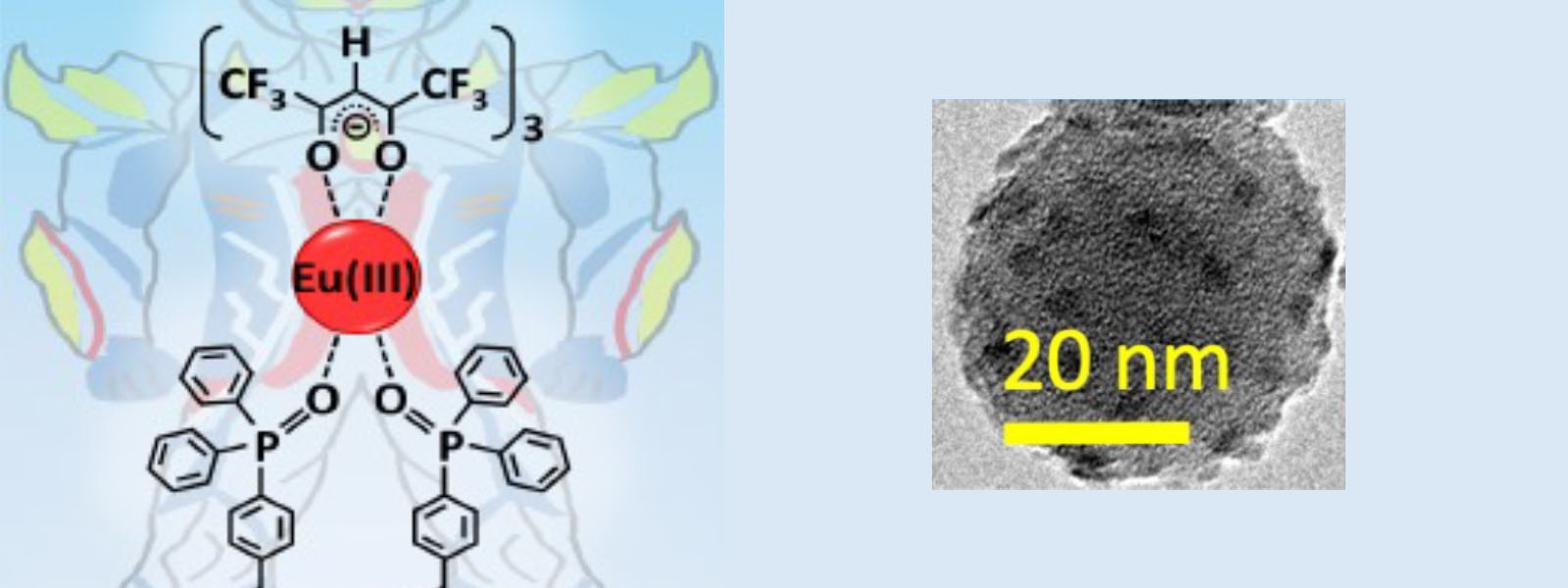 A joint paper with Prof. Hasegawa. |
| 69. | Calix[3]pyrrole: A Missing Link in Porphyrin-Related Chemistry Y. Inaba, Y. Nomata, Y. Ide, J. Pirillo, Y. Hijikata, T. Yoneda, A. Osuka, J. L. Sessler, Y. Inokuma J. Am. Chem. Soc. 2021, 143, 12355-12360. 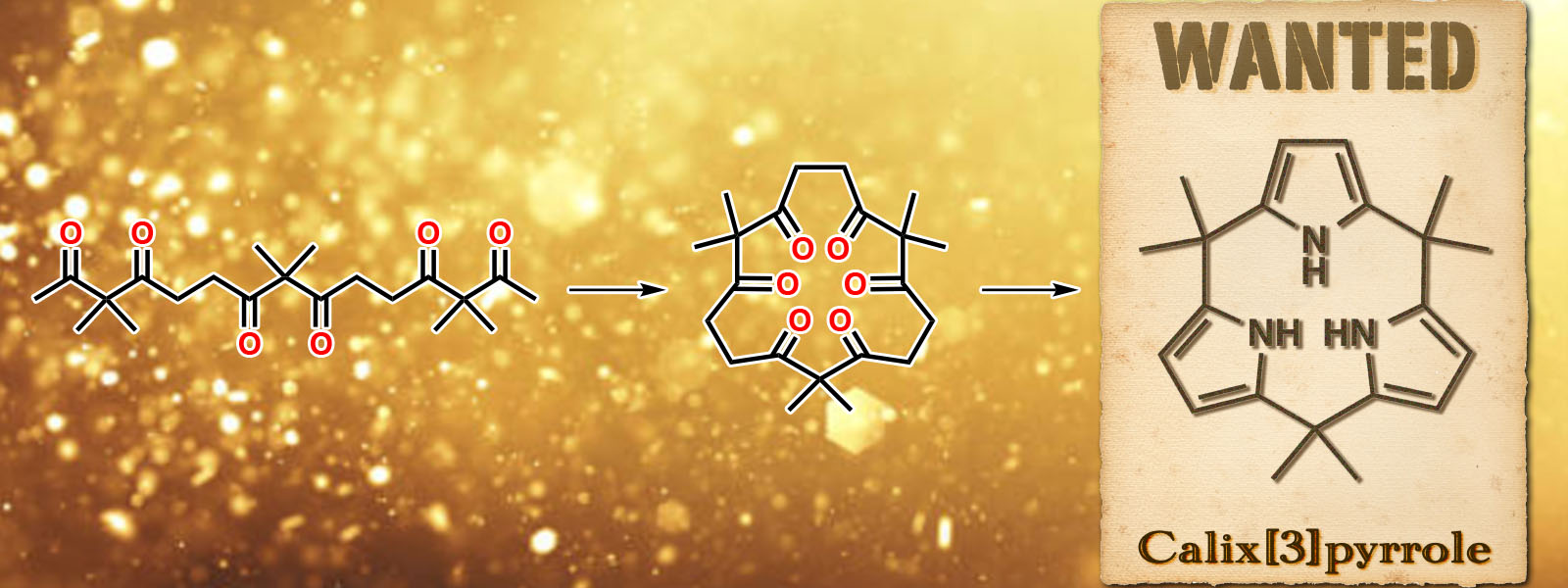 Calix[3]pyrrole was finally synthesized from a carbonyl rope. Highlighted by Science "In Other Journals". Highlighted by Synfacts Press release (Hokkaido University) |
| 68. | Isopyrazole-Masked Tetraketone: Tautomerism and Functionalization for Fluorescent Metal Ligands H. Shirakura, Y. Manabe, C. Kasai, Y. Inaba, M. Tsurui, Y. Kitagawa, Y. Hasegawa, T. Yoneda, Y. Ide, Y. Inokuma Eur. J. Org. Chem. 2021, 4345–4349. 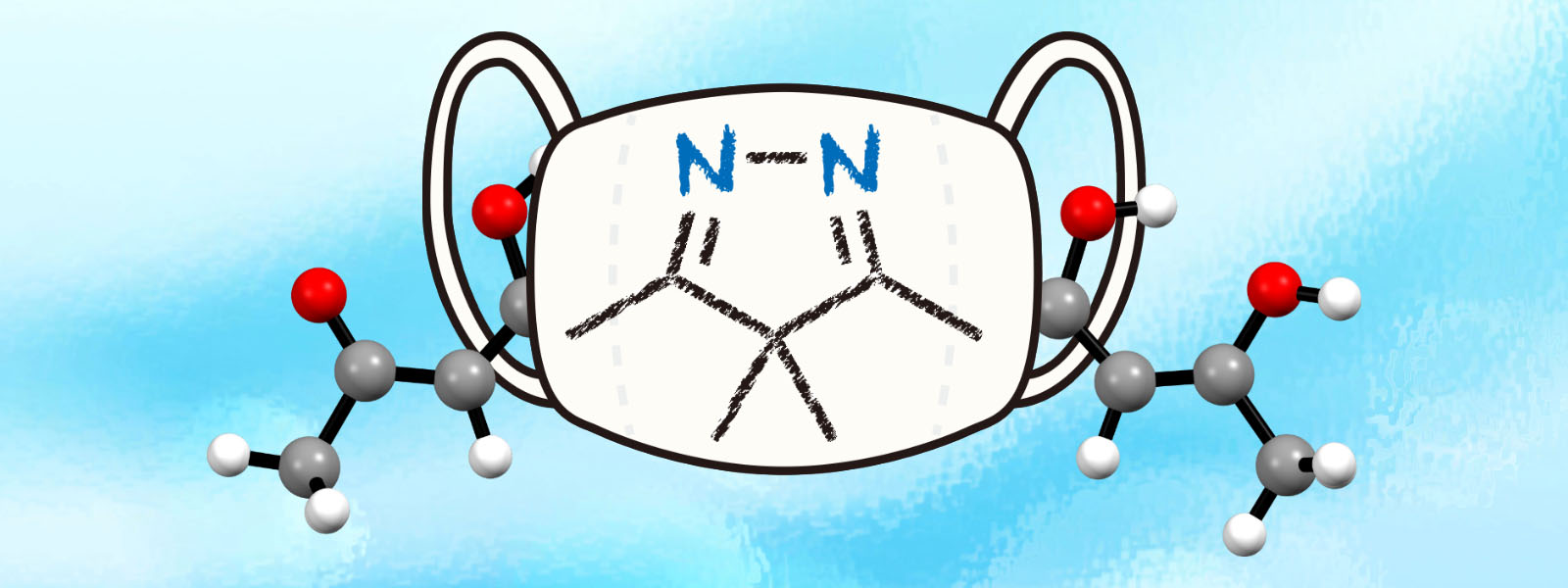 Fluorescent metal ligand was synthesized by isopyrazole-masking strategy. |
| 67. | Polyketone-Based Molecular Ropes as Versatile Components for Functional Materials Y. Inokuma, Y. Inaba Bull. Chem. Soc. Jpn. 2021, 94, 2187-2194. 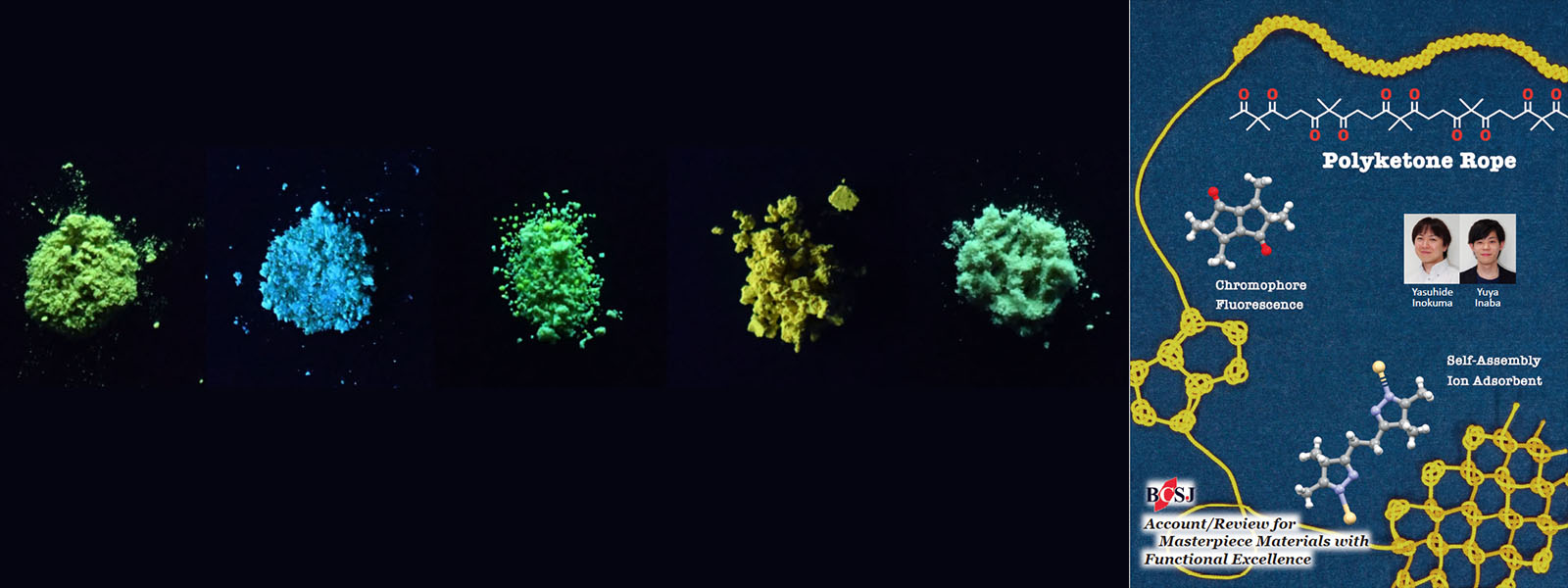 BCSJ Account for application of carbonyl ropes as a base component for functional molecular materials. |
| 66. | Insoluble π‐Conjugated Polyimine as an Organic Adsorbent for Group 10 Metal Ions H. Shirakura, Y. Hijikata, J. Pirillo, T. Yoneda, Y. Manabe, M. Murugavel, Y. Ide, Y. Inokuma Eur. J. Inorg. Chem. 2021, 1705-1708. 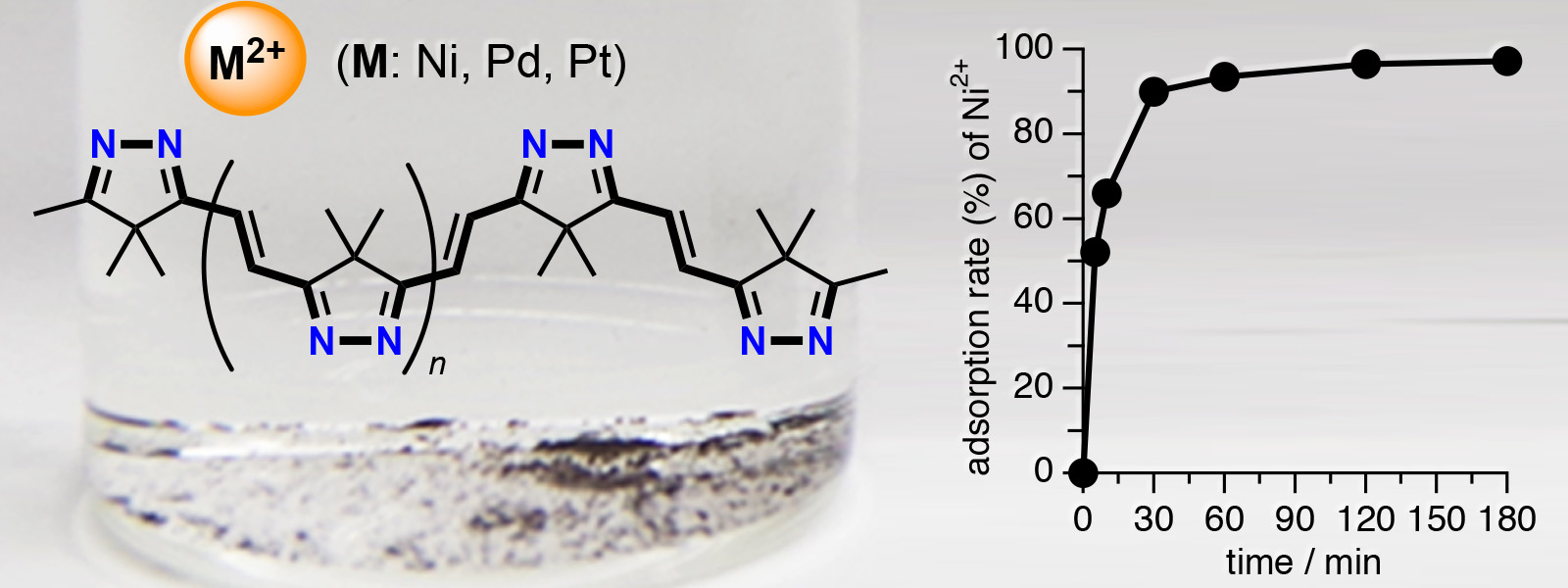 Metal-ion adsorbent property of conjugated polyimines generated from aliphatic polyketones. |
| 65. | Aliphatic Polyketones as Classic yet New Molecular Ropes for Structural Diversity in Organic Synthesis Y. Inokuma, T. Yoneda, Y. Ide, S. Yoshioka Chem. Commun., 2020, 56, 9079-9093. 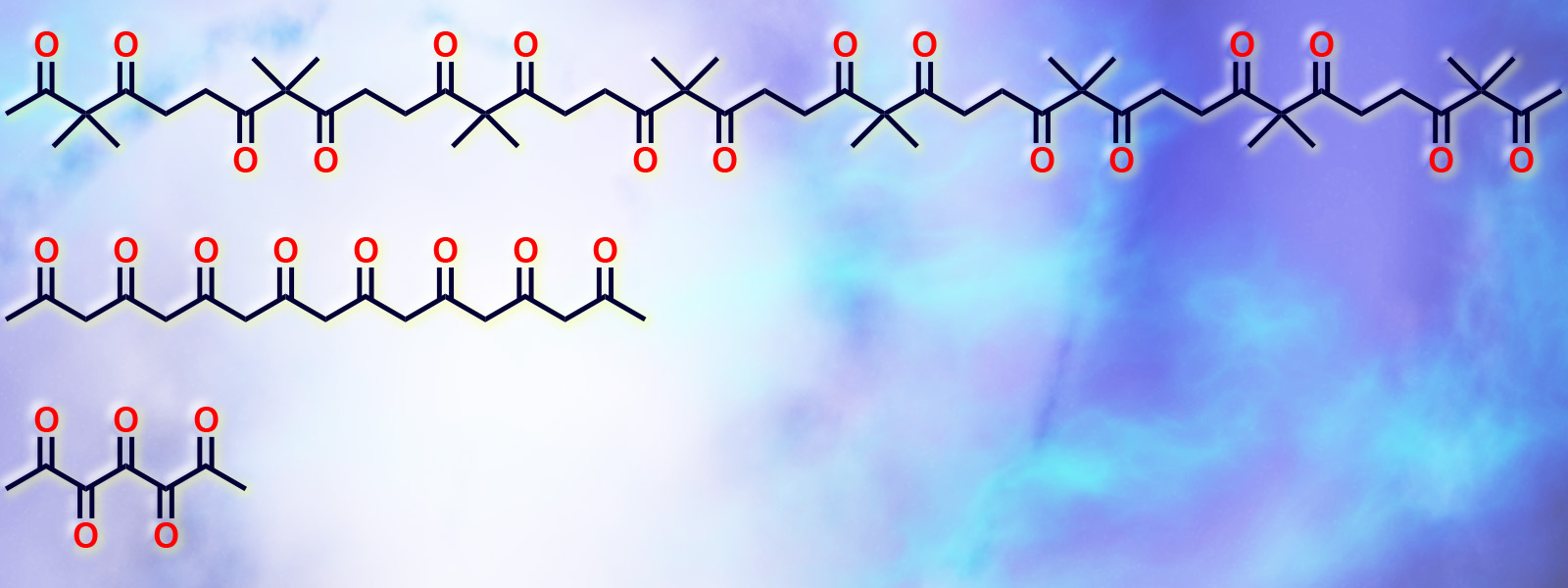 Feature Article of recent developments in carbonyl ropes chemistry. |
| 64. | Spirosilicate Dimers of a Bowl-shaped Diol Generated by Intramolecular Cyclization of an Aliphatic Tetraketone Chains Y. Inaba, Y. Inokuma Chem. Lett. 2020, 49, 882-884. 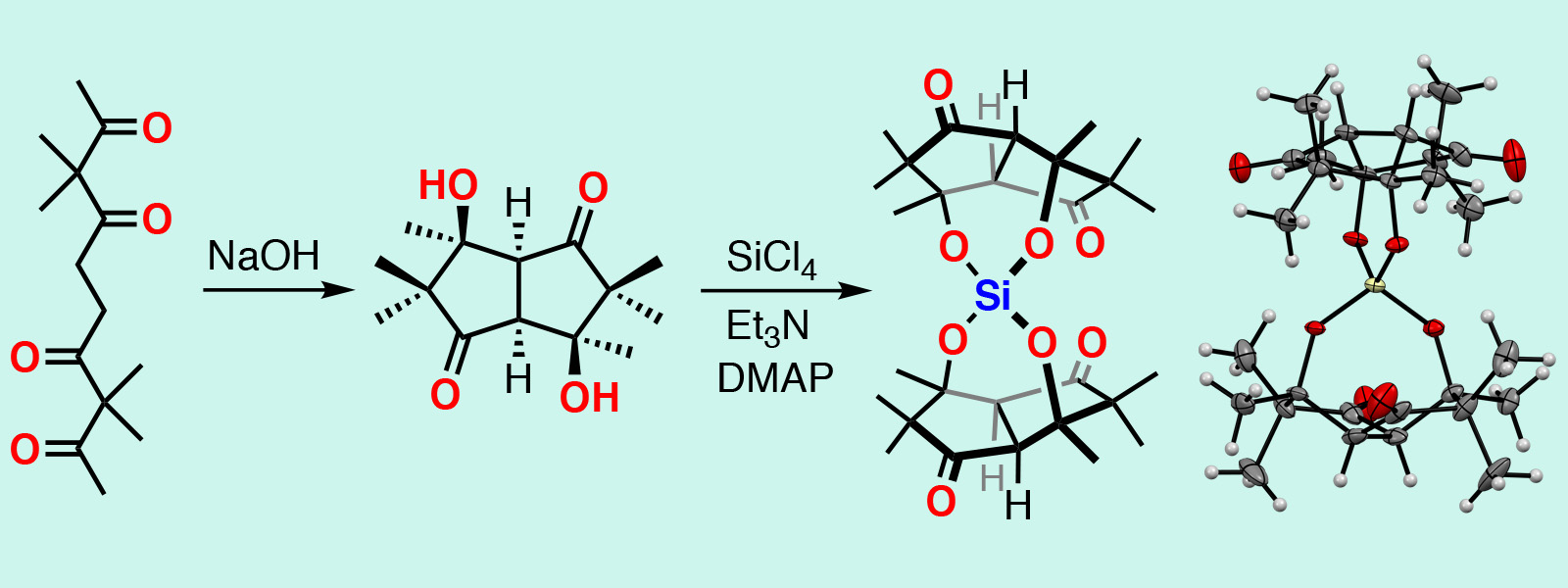 Synthesis of hydrolysis-stable spirosilicates using a bowl-shaped diol generated by intramolecular cyclization of carbonyl a rope. |
| 63. | Modular Synthesis of Oligoacetylacetones via Site-selective Silylation of Acetylacetone Derivatives P. Sarkar, Y. Inaba, H. Shirakura, T. Yoneda, Y. Inokuma Org. Biomol. Chem. 2020, 18, 3297-3302. 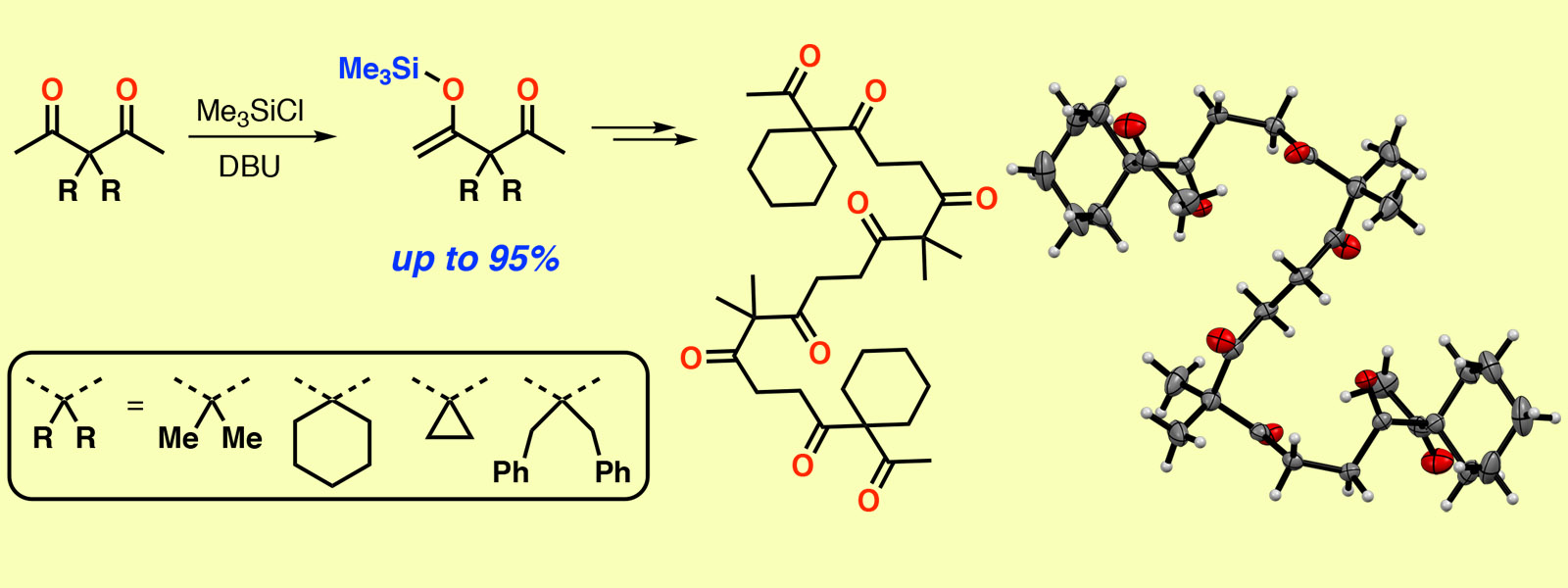 Modular synthesis of carbonyl ropes enables to prepare aliphatic oligoketones with various substituent sequences. |
| 62. | Supramolecular Conformational Control of Aliphatic Oligoketones by Rotaxane Formation Y. Manabe, K. Wada, Y. Baba, T. Yoneda, T. Ogoshi, Y. Inokuma Org. Lett. 2020, 22, 3224-3228. 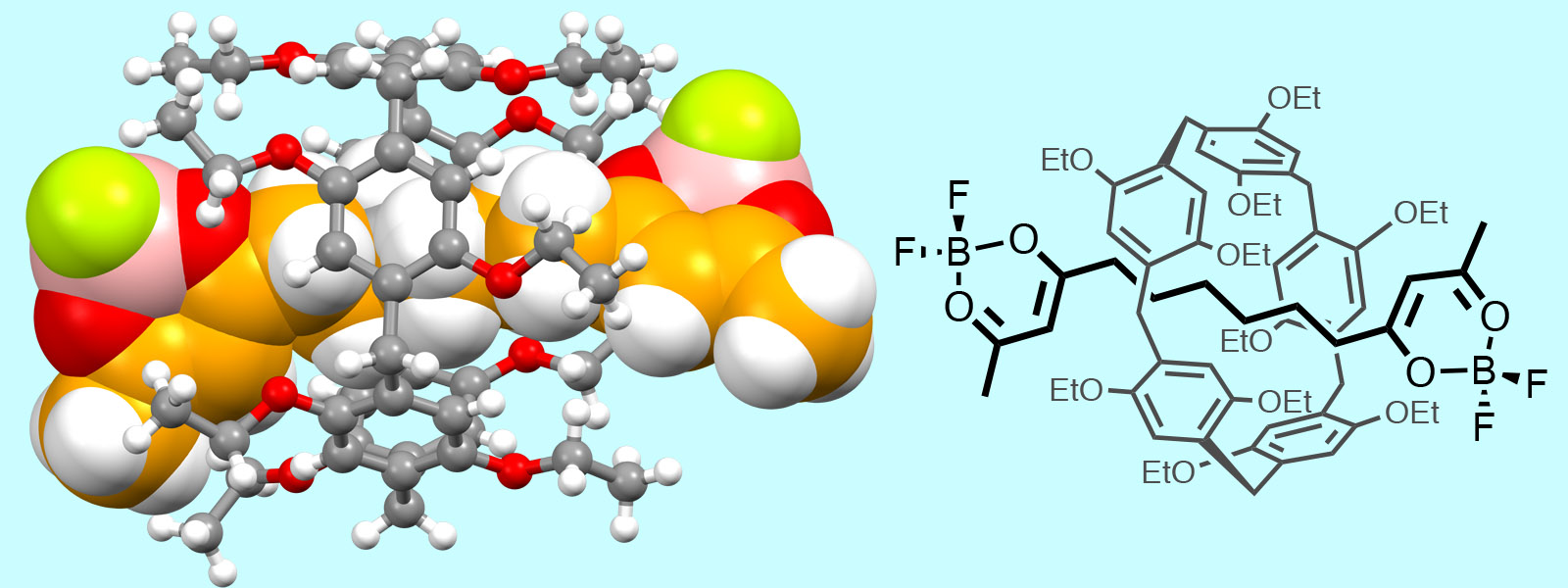 Rotaxane formation between pillar[5]arene and carbonyl rope. Collaborative research with Prof. Ogoshi group at Kyoto Univ. |
| 61. | Chiral Monolayers with Achiral Tetrapod Molecules on Highly Oriented Pyrolytic Graphite H. Asakawa, S. Matsui, Q. T. Trinh, H. Hirao, Y. Inokuma, T. Ogoshi, S. Tanaka, K. Komatsu, A. Ohta, T. Asakawa, T. Fukuma J. Phys. Chem. C 2020, 124, 7760-7767. 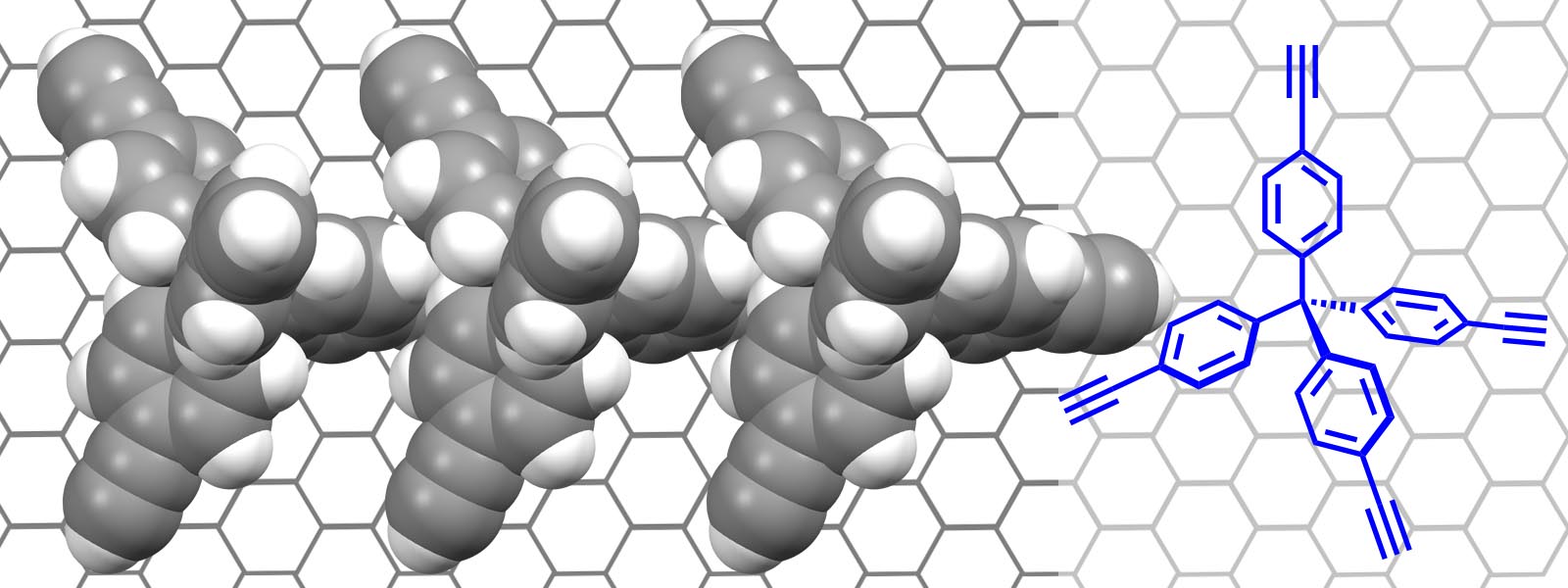 Collaboration research paper with PRESTO "Hyper-nano Space Design" and Kanazawa Univ. WPI-NanoLSI members on orientation of tetrapod molecules on HOPG surface. |
| 60. | Polyketones as Host Materials for Solid Polymer Electrolytes T. Eriksson, A. Mace, Y. Manabe, M. Yoshizawa-Fujita, Y. Inokuma, D. Brandell, J. Mindemark J. Electrochem. Soc. 2020, 167, 070537. 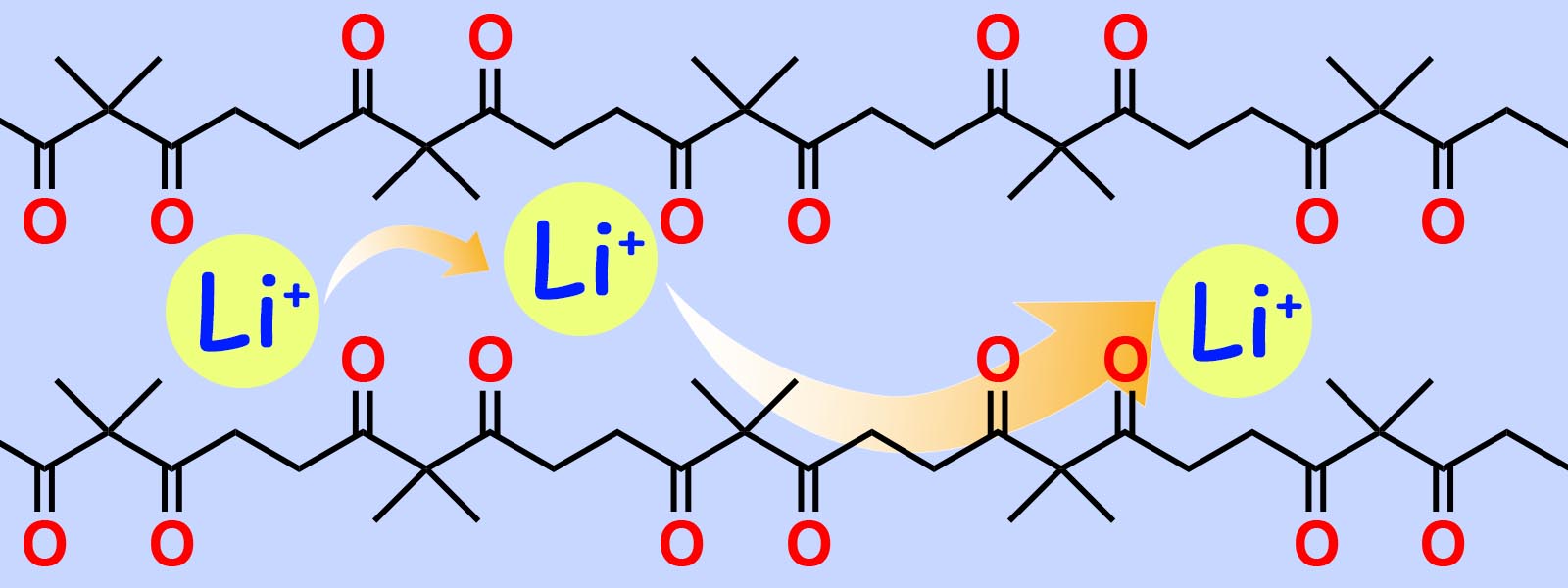 International joint research on application of polyketones for solid electrolytes with Dr. Jonas Mindemark (Uppsala Univ.) and Prof. Masahiro Yoshizawa-Fujita (Sophia Univ.). |
| 59. | Luminescent Coordination Polymers Constructed from Flexible, Tetradentate Diisopyrazole Ligand and Copper(I) Halides T. Yoneda, C. Kasai, Y. Manabe, M. Tsurui, Y. Kitagawa, Y. Hasegawa, P. Sarkar, Y. Inokuma Chem. Asian J. 2020, 15, 601-605. 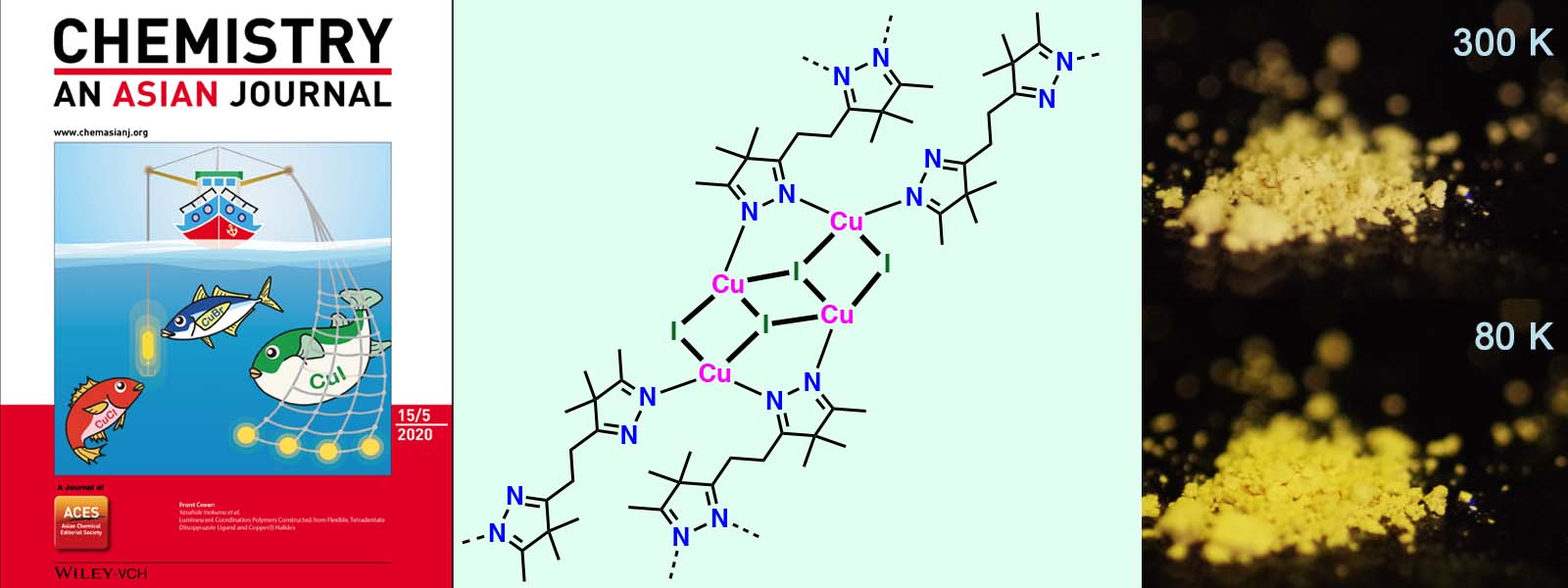 Luminescent coordination polymers generated from an aliphatic imine ligand and Cu(I) halides. Thermochromic behavior was observed for 2-dimensional Cu4I4 cluster network. (Selected as Front Cover) |
| 58. | Splitting and Reorientation of π-Conjugation by an Unprecedented Photo-Rearrangement Reaction Y. Inaba, T. Yoneda, Y. Kitagawa, K. Miyata, Y. Hasegawa, Y. Inokuma Chem. Commun. 2020, 56, 348-351. 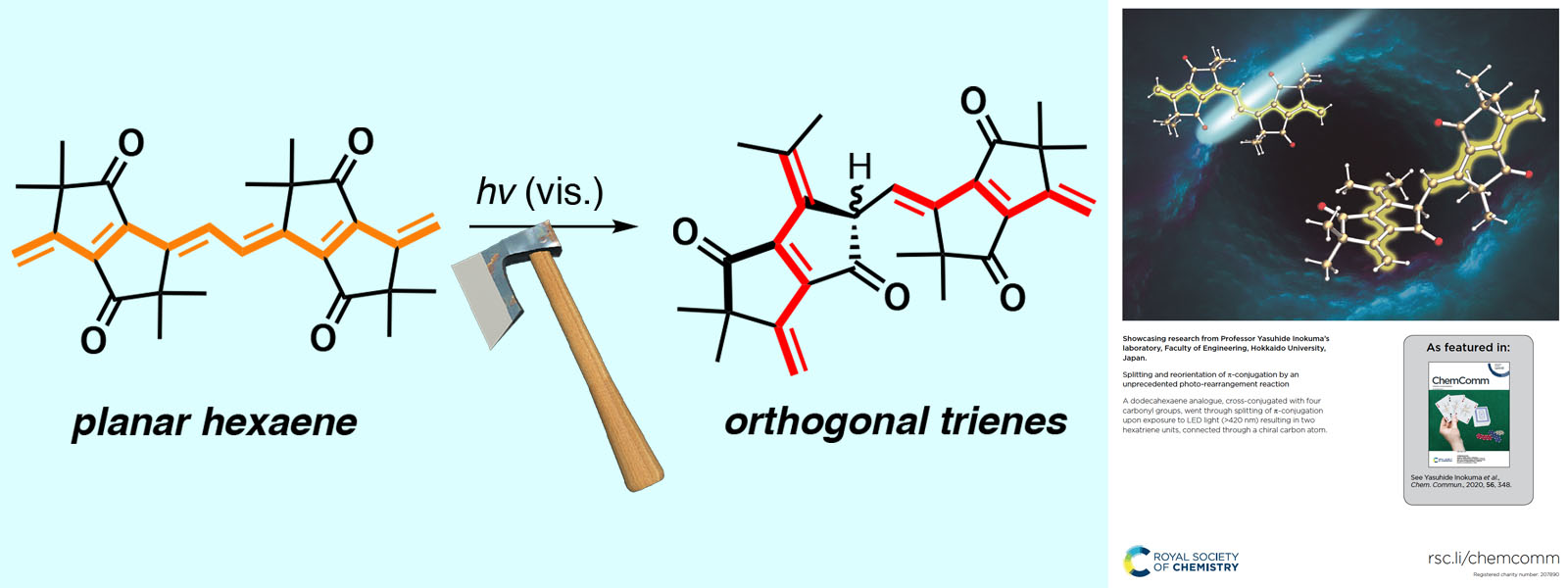 Nobel photo-induced rearrangement reaction for splitting π-conjugation of polyene. (Selected as Back Cover) |
| 57. | Identification of Actinomycin D as a Specific Inhibitor of the Alternative Pathway of Peptidoglycan Biosynthesis Y. Ogasawara, Y. Shimizu, Y. Sato, T. Yoneda, Y. Inokuma, T. Dairi J. Antibiot. 2020, 73, 125-127. 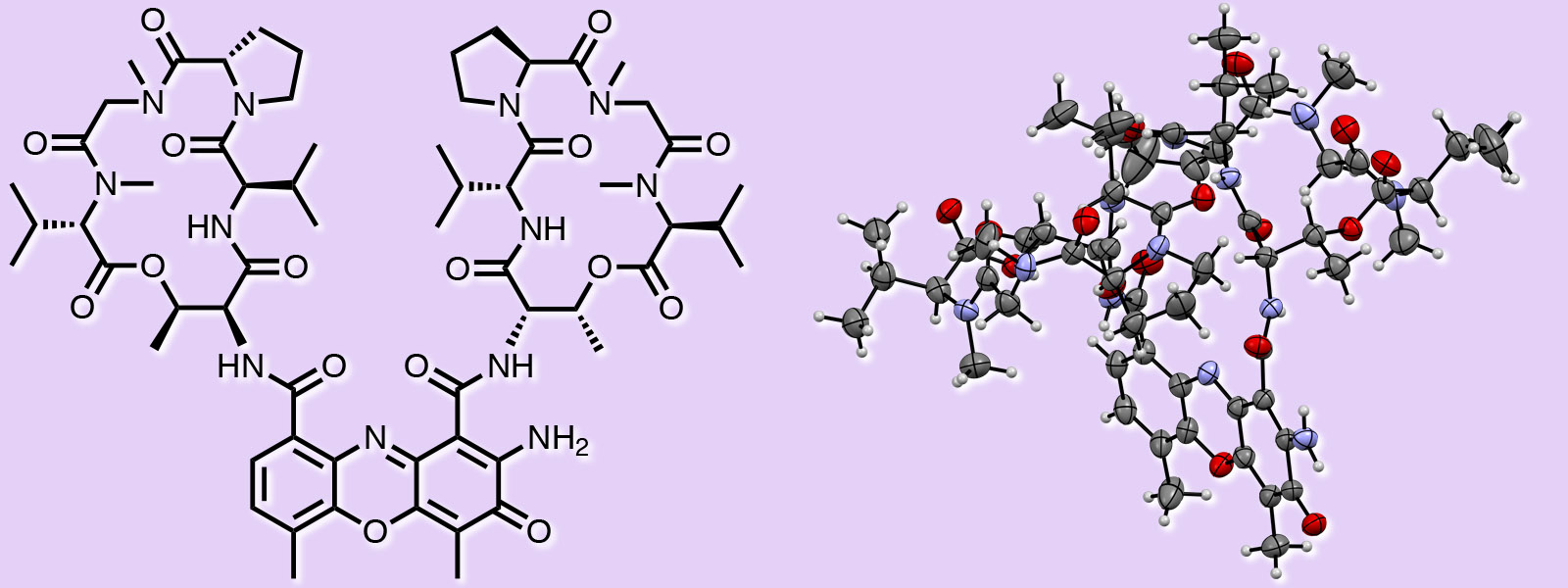 A joint paper with Dr. Ogasawara and Prof. Dairi. |
| 56. | Asymmetric Synthesis of a 5,7-Fused Ring System Enabled by an Intramolecular Buchner Reaction with Chiral Rhodium Catalyst T. Hoshi, E. Ota, Y. Inokuma, J. Yamaguchi Org. Lett. 2019, 21, 10081-10084. 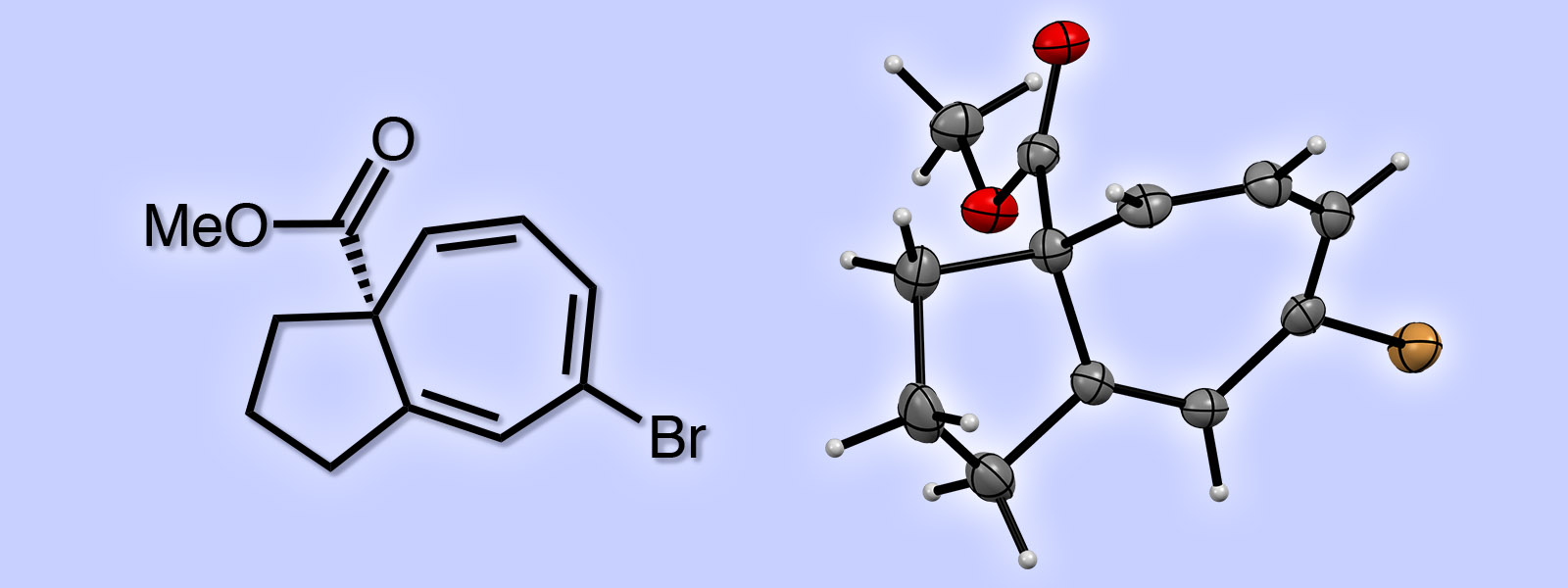 A joint paper with Prof. Yamaguchi (Waseda Univ.). |
| 55. | Aliphatic Polyketones as Shapable Molecular Chains Y. Inokuma J. Synth. Org. Chem. Jpn. 2019, 77, 1078-1085. 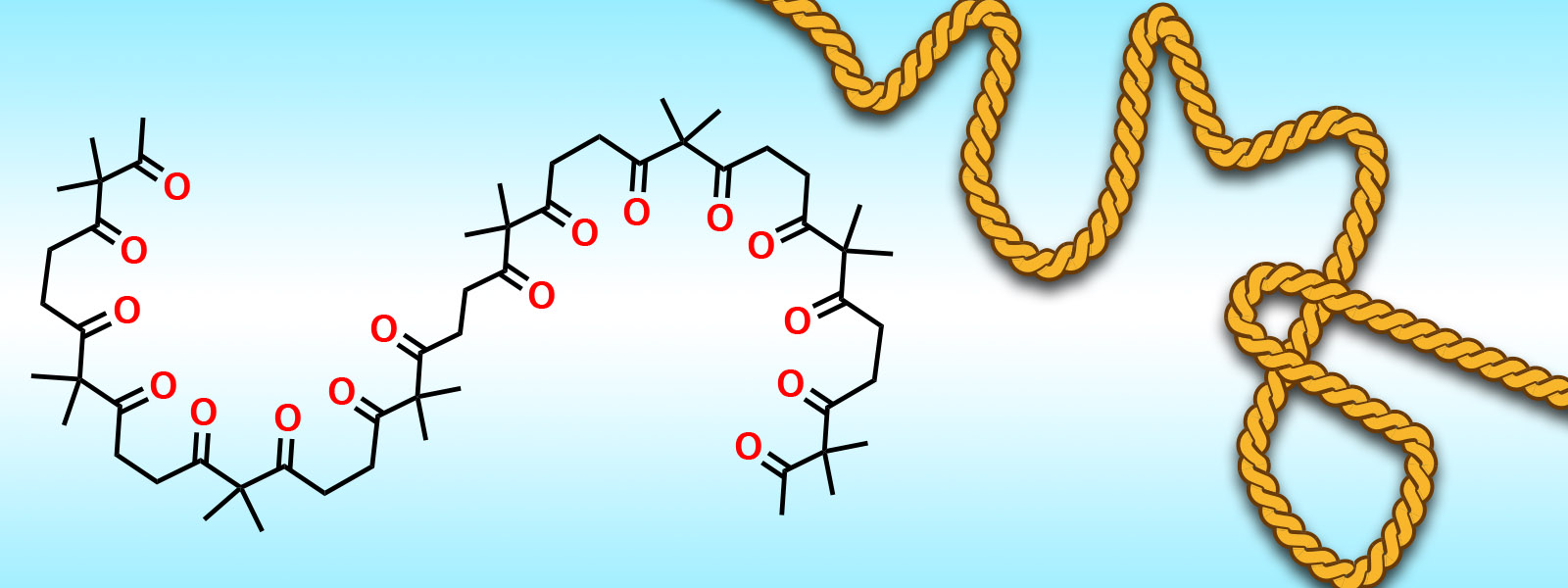 An account of carbonyl ropes. |
| 54. | Two-Step Transformation of Aliphatic Polyketones into π-Conjugated Polyimines Y. Manabe, M. Uesaka, T. Yoneda, Y. Inokuma J. Org. Chem. 2019, 84, 9957-9964. 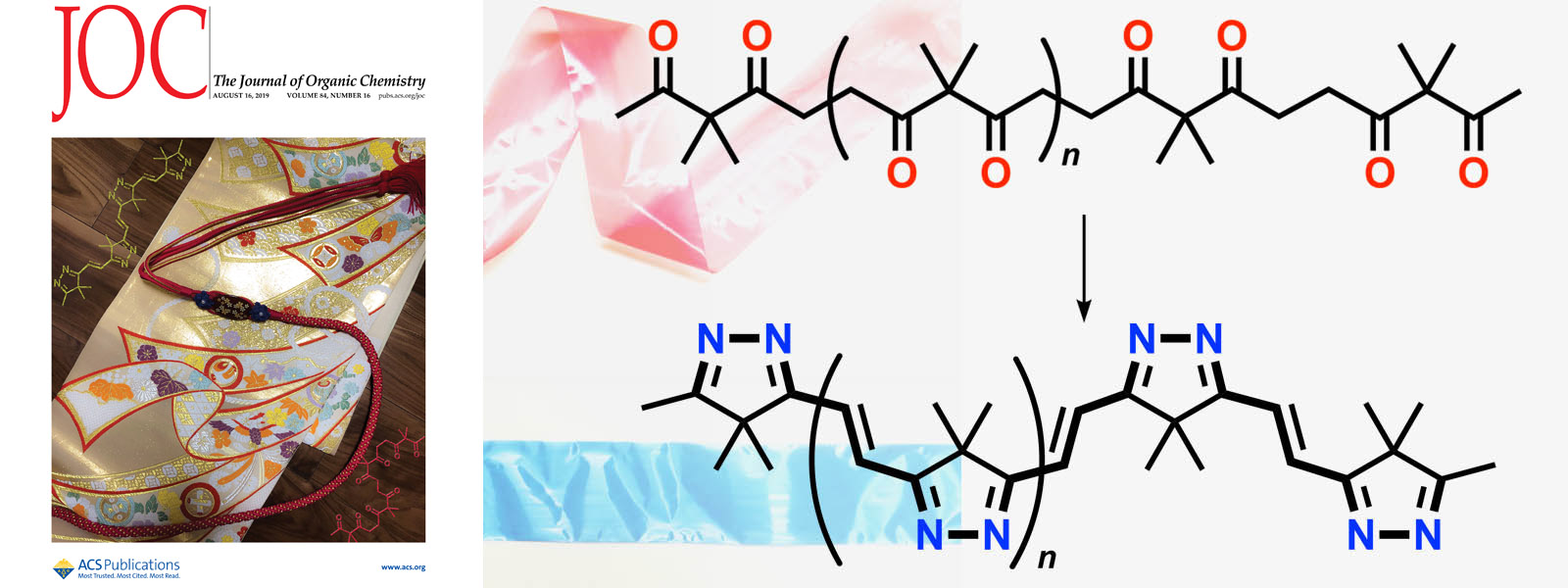 Carbonyl ropes were converted into highly π-conjugated imine 'sashes'. (Selected as Cover Picture) |
| 53. | Control over Coordination Self-Assembly of Flexible, Multidentate Ligands by Stepwise Metal Coordination of Isopyrazole Subunits Y. Ashida, Y. Manabe, S. Yoshioka, T. Yoneda, Y. Inokuma Dalton Trans. 2019, 48, 818-822. 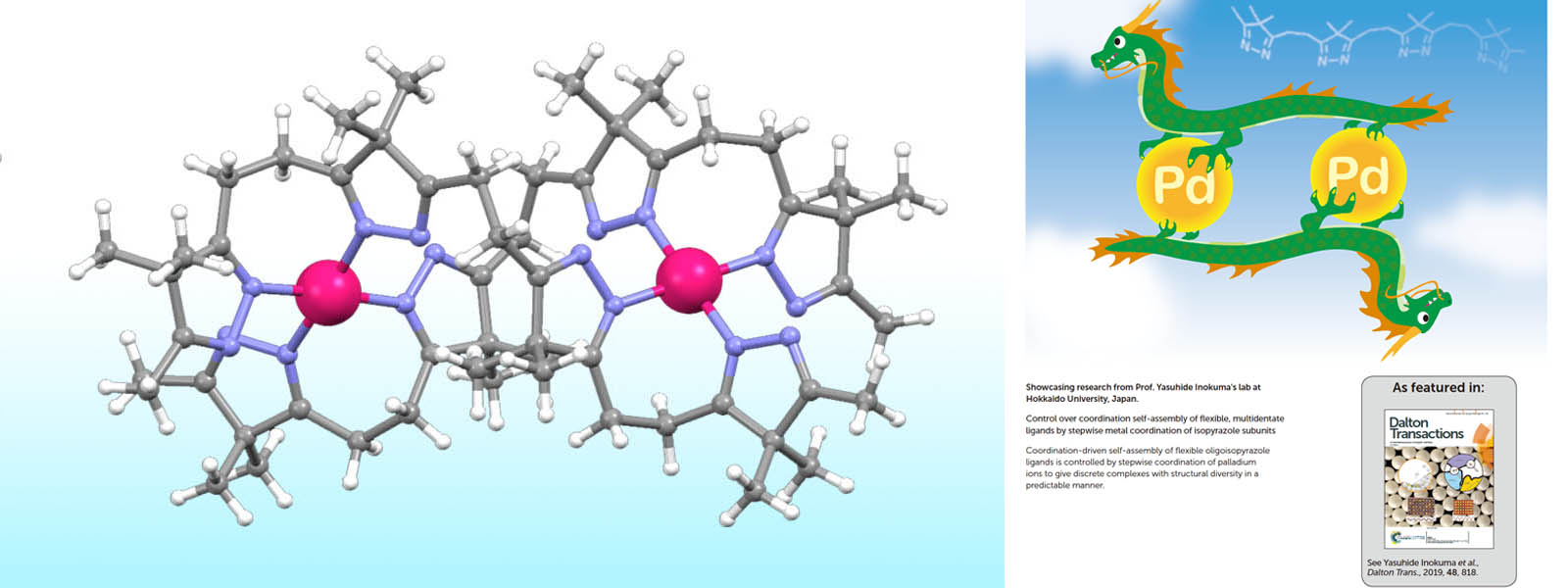 Self-assembly of oligoimine chains by the aid of stepwise palladium ion coordination. (Selected as Inside Back Cover) |
| 52. | Bioinspired Synthesis of Pentalene-based Chromophores from an Oligoketone Chain Y. Saito, M. Higuchi, S. Yoshioka, H. Senboku, Y. Inokuma Chem. Commun. 2018, 54, 6788-6791. 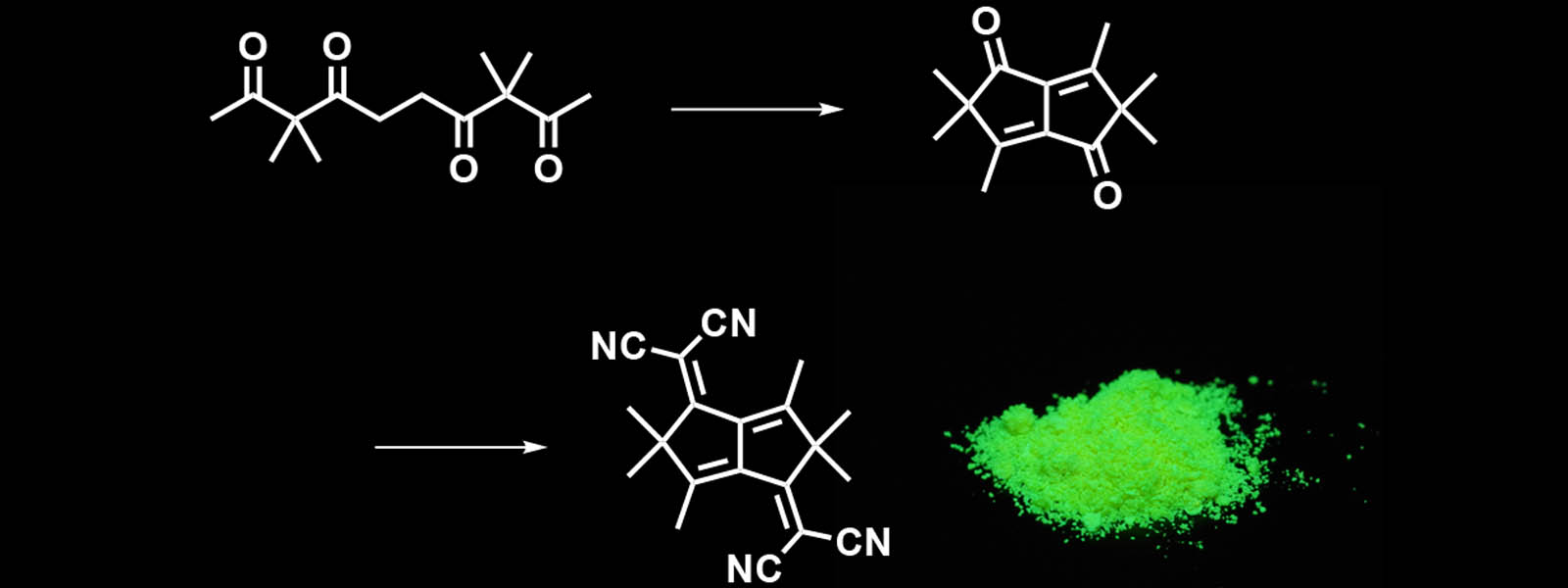 Folding a carbonyl string to make chromophores that absorb and emit visible light. |
| 51. | Oligoacetylacetones as Shapable Carbon Chains and Their Transformation to Oligoimines for Construction of Metal-organic Architectures M. Uesaka, Y. Saito, S. Yoshioka, Y. Domoto, M. Fujita, Y. Inokuma Communications Chemistry 2018, 1, Article Number 23.  Our first paper from Hokkaido University!! The concept, synthesis and transformation of polycarbonyl chains are reported. Behind the Paper (Nature Research Chemistry Community) |
| 50. | Chiral Crystalline Sponges for the Absolute Structure Determination of Chiral Guests K. Yan, R. Dubey, T. Arai, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2017, 139, 11341-11344. |
| 49. | Structural Elucidation of Trace Amounts of Volatile Compounds Using the Crystalline Sponge Method N. Zigon, T. Kikuchi, J. Ariyoshi, Y. Inokuma, M. Fujita Chem. Asian J. 2017, 12, 1057-1061. |
| 48. | Finding a New Crystalline Sponge from a Crystallographic Database Y. Inokuma, K. Matsumura, S. Yoshioka, M. Fujita Chem. Asian. J. 2017, 12, 208-211. (Front Cover) |
| 47. | X-ray Structure Analysis of Ozonides by the Crystalline Sponge Method S. Yoshioka, Y. Inokuma, V. Duplan, R. Dubey, M. Fujita J. Am. Chem. Soc. 2016, 138, 10140-10142. |
| 46. | A Saccharide-based Crystalline Sponge for Hydrophilic Guests G.-H. Ning, K. Matsumura, Y. Inokuma, M. Fujita Chem. Commun. 2016, 52, 7013-7015. |
| 45. | Structure Determination of Microbial Metabolites by the Crystalline Sponge Method Y. Inokuma, T. Ukegawa, M. Hoshino, M. Fujita Chem. Sci. 2016, 7, 3910-3913. |
| 44. | The Crystalline Sponge Method Updated M. Hoshino, A. Khutia, H. Xing, Y. Inokuma, M. Fujita IUCrJ 2016, 3, 139-151. |
| 43. | Where is the Oxygen? Structural Analysis of a-Humulene Oxidation Products by the Crystalline Sponge Method N. Zigon, M. Hoshino, S. Yoshioka, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2015, 54, 9033-9037. |
| 42. | Absolute Structure Determination of Compounds with Axial and Planar Chirality Using the Crystalline Sponge Method S. Yoshioka, Y. Inokuma, M. Hoshino, T. Sato, M. Fujita Chem. Sci. 2015, 6, 3765-3768. |
| 41. | Networked-Cage Microcrystals for Evaluation of Host-guest Interactions S. Matsuzaki, T. Arai, K. Ikemoto, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2014, 136, 17899-17901. |
| 40. | Visualization of Solution Chemistry by X-ray Crystallography Using Porous Coordination Networks Y. Inokuma, M. Fujita Bull. Chem. Soc. Jpn. 2014, 87, 1161-1176. (Award Article, Back Cover) |
| 39. | Radical C-H Functionalization of Heteroarenes under Electrochemical Control A. O'Brien, A. Maruyama, Y. Inokuma, M. Fujita, P. S. Baran, D. G. Blackmond Angew. Chem. Int. Ed. 2014, 53, 11868-11871. (Selected as a Hot Paper) |
| 38. | X-ray Snapshot Observation of Palladium-Mediated Aromatic Bromination in a Porous Complex K. Ikemoto, Y. Inokuma, K. Rissanen, M. Fujita J. Am. Chem. Soc. 2014, 136, 6892-6895. (Highlighted by "Chemistry World", RSC) |
| 37. | Preparation and Guest-uptake Protocol for a Porous Complex Useful for 'Crystal-free' Crystallography Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, M. Fujita Nature Protocols 2014, 9, 246-252. |
| 36. | Stable Encapsulation of Acrylate Esters in Networked Molecular Capsules G.-H Ning, Y. Inokuma, M. Fujita Chem. Asian J. 2014, 9, 466-468. (VIP paper, Inside Cover) |
| 35. | Unique Ultrafast Energy Transfer in a Series of Phenylene-bridged Subporphyrin-porphyrin Hybrids J. Oh, J. Sung, M. Kitano, Y. Inokuma, A. Osuka, D. Kim Chem. Commun. 2014, 50, 10424-10426. |
| 34. | Dynamic Behavior of M6L4 Capsules in Solution and Crystalline States G.-H Ning, Y. Inokuma, M. Fujita Chem. Asian J. 2013, 8, 2596-2599. |
| 33. | X-ray analysis on the nanogram to microgram scale using porous complexes Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen, M. Fujita Nature 2013, 495, 461-466. (Highlighted by "Nature news and views" ) (Highlighted by "C&EN", ACS) (Highlighted by "Chemistry World", RSC) |
| 32. | Reagent-Installed Capsule Network: Selective Thiocarbamoylation of Aromatic Amines in Crystals with Pre-installed CH3NCS Y. Inokuma, G.-H. Ning, M. Fujita Angew. Chem. Int. Ed. 2012, 51, 2379-2381. |
| 31. | Oxocyclohexadienylidene-Substituted Subporphyrins S. Hayashi, J. Sung, Y. Sung, Y. M. Sung, Y. Inokuma, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2011, 50, 3253-3256. |
| 30. | Bimolecular Reaction via the Successive Introduction of Two Substrates into the Crystals of Networked Molecular Cages Y. Inokuma, N. Kojima, T. Arai, M. Fujita J. Am. Chem. Soc. 2011, 133, 19691-19693. |
| 29. | Diels-Alder via Molecular Recognition in a Crystalline Molecular Flask K. Ikemoto, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2011, 133, 16806-16808. |
| 28. | Shedding Light on Hidden Reaction Pathways in Radical Polymerization by a Porous Coordination Network Y. Inokuma, S. Nishiguchi, K. Ikemoto, M. Fujita Chem. Commun. 2011, 47, 12113-12115. |
| 27. | Synthesis and Properties of Boron(III)-Coordinated Subbacteriochlorins S. Hayashi, E. Tsurumaki, Y. Inokuma, P. Kim, Y. M. Sung, D. Kim, A. Osuka J. Am. Chem. Soc. 2011, 133, 4254-4256. |
| 26. | Crystalline Molecular Flasks Y. Inokuma, M. Kawano, M. Fujita Nature Chem. 2011, 3, 349-358. |
| 25. | A Molecular Capsule Network: Guest Encapsulation and Control of Diels-Alder Reactivity Y. Inokuma, S. Yoshioka, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 8912-8914. |
| 24. | Networked Molecular Cages as Crystalline Sponges for Fullerenes and Other Guests Y. Inokuma, T. Arai, M. Fujita Nature Chem. 2010, 2, 780-783. (Highlighted by "C&EN", ACS) |
| 23. | The Reaction of Organozinc Compounds with an Aldehyde within a Crystalline Molecular Flask K. Ikemoto, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 5750-5752. |
| 22. | The Catalytic Z to E Isomerization of Stilbenes in a Photosensitizing Porous Coordination Network K. Ohara, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 5507-5509. |
| 21. | Regioselective Huisgen Cycloaddition within Porous Coordination Networks T. Kawamichi, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 2375-2377. (Highlighted by "Noteworthy Chemistry", ACS) |
| 20. | A Porous Coordination Network Catalyzes an Olefin Isomerization Reaction in the Pore K. Ohara, M. Kawano, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2010, 132, 30-31. |
| 19. | meso-Tris(oligo-2,5-thienylene)-Substituted Subporphyrins S. Hayashi, Y. Inokuma, A. Osuka Org. Lett. 2010, 12, 4148-4151. |
| 18. | meso-Trifluoromethyl-substituted Subporphyrin from Ring-splitting Reaction of meso-Trifluoromethyl-substituted [32]Heptaphyrin(1.1.1.1.1.1.1) R. Sakamoto, S. Saito, S. Shimizu, Y. Inokuma, N. Aratani, A. Osuka Chem. Lett. 2010, 39, 439-441. |
| 17. | meso-Trialkyl-substituted Subporphyrins S. Hayashi, Y. Inokuma, S. Easwaramoorthi, K. S. Kim, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2010, 49, 321-324. |
| 16. | Versatile Photophysical Properties of meso-Aryl Substituted Subporphyrins: Dipolar and Octupolar Charge-Transfer Interactions S. Easwaramoorthi, J.-Y. Shin, S. Cho, P. Kim, Y. Inokuma, E. Tsurumaki, A. Osuka, D. Kim Chem. Eur. J. 2009, 15, 12005-12017. |
| 15. | Capped Subporphyrins Y. Inokuma, A. Osuka Chem. Eur. J. 2009, 15, 6863-6876. |
| 14. | 1,4-Phenylene-bridged Subporphyrin-porphyrin Dyad, Triad, and Tetrad Y. Inokuma, S. Hayashi, A. Osuka Chem. Lett. 2009, 38, 206-207. |
| 13. | Peripheral Hexabromination, Hexaphenylation, and Hexaethynylation of meso-Aryl- Substituted Subporphyrins E. Tsurumaki, Y. Inokuma, S. Easwaramoorthi, J. M. Lim, D. Kim, A. Osuka Chem. Eur. J. 2009, 15, 237-247. |
| 12. | 3,3- and 4,4-Biphenylene-Bridged Subporphyrin Dimers Y. Inokuma, A. Osuka Org. Lett. 2008, 10, 5561-5564. |
| 11. | Unambiguous Identification of Möbius Aromaticity for meso-Aryl-Substituted [28]Hexaphyrins(1.1.1.1.1.1) J. Sankar, S. Mori, S. Saito, H. Rath, M. Suzuki, Y. Inokuma, H. Shinokubo, K. S. Kim, Z. S. Yoon, J.-Y. Shin, J. M. Lim, Y. Matsuzaki, O. Matsushita, A. Muranaka, N. Kobayashi, D. Kim, A. Osuka J. Am. Chem. Soc. 2008, 130, 13568-13579. |
| 10. | meso-(4-(N,N-Dialkylamino)phenyl)-Substituted Subporphyrins: Remarkably Perturbed Absorption Spectra and Enhanced Fluorescence by Intramolecular Charge Transfer Interactions Y. Inokuma, S. Easwaramoorthi, Z. S. Yoon, D. Kim, A. Osuka J. Am. Chem. Soc. 2008, 130, 12234-12235. |
| 9. | Effective Expansion of the Subporphyrin Chromophore Through Conjugation with meso-Oligo(1,4-phenyleneethynylene) Substituents: Octupolar Effect on Two-Photon Absorption Y. Inokuma, S. Easwaramoorthi, S. Y. Jang, K. S. Kim, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2008, 47, 4840-4843. |
| 8. | Subporphyrins: Emerging Contracted Porphyrins with Aromatic 14π-Electronic Systems and Bowl-Shaped Structures: Rational and Unexpected Synthetic Routes Y. Inokuma, A. Osuka Dalton Trans. 2008, 2517-2526. (Perspective, Front Cover) |
| 7. | Synthesis and Characterization of meso-Aryl-Substituted Subchlorins E. Tsurumaki, S. Saito, K. S. Kim, J. M. Lim, Y. Inokuma, D. Kim, A. Osuka J. Am. Chem. Soc. 2008, 130, 438-439. |
| 6. | Complementary Face-to-Face Dimer Formation From meso-Aryl Subporphyrins Bearing a 2-Carboxyphenyl Group Y. Inokuma, A. Osuka Chem. Commun. 2007, 2938-2940. |
| 5. | meso-Aryl-Substituted Subporphyrins: Synthesis, Structures, and Large Substituent Effects on Their Electronic Properties Y. Inokuma, Z. S. Yoon, D. Kim, A. Osuka J. Am. Chem. Soc. 2007, 129, 4747-4761. |
| 4. | Tribenzosubporphines: Synthesis and Characterization Y. Inokuma, J. H. Kwon, T. K. Ahn, M.-C. Yoon, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2006, 45, 961-964. |
| 3. | Enlarged π-Electronic Network of a meso-meso, β-β, β-β Triply Linked Dibenzoporphyrin Dimer that Exhibits a Large Two-Photon Absorption Cross Section Y. Inokuma, N. Ono, H. Uno, D. Y. Kim, S. B. Noh, D. Kim, A. Osuka Chem. Commun. 2005, 3782-3784. |
| 2. | A Doubly N-Fused Benzohexaphyrin and Its Rearrangement to a Fluorescent Macrocycle upon DDQ Oxidation Y. Inokuma, T. Matsunari, N. Ono, H. Uno, A. Osuka Angew. Chem. Int. Ed. 2005, 44, 1856-1860. |
| 1. | meso-Porphyrinyl-Substituted Porphyrin and Expanded Porphyrins Y. Inokuma, A. Osuka Org. Lett. 2004, 6, 3663-3666. |
