Research
It's a Small 'Porphyrin' World!
Porphyrins are tetrapyrrolic macrocycles commonly found in heme and chlorophyll. A long-standing mystery in both laboratory and natural porphyrin syntheses is that “pyrrole monomers selectively form tetrapyrrolic macrocycles, but those with three pyrrole monomers are never observed.” Calix[3]pyrrole, a porphyrinogen-like macrocycle bearing three pyrrole units, may hold the key to this mystery, but has been elusive in porphyrin-related chemistry.
The mystery behind porphyrin synthesis
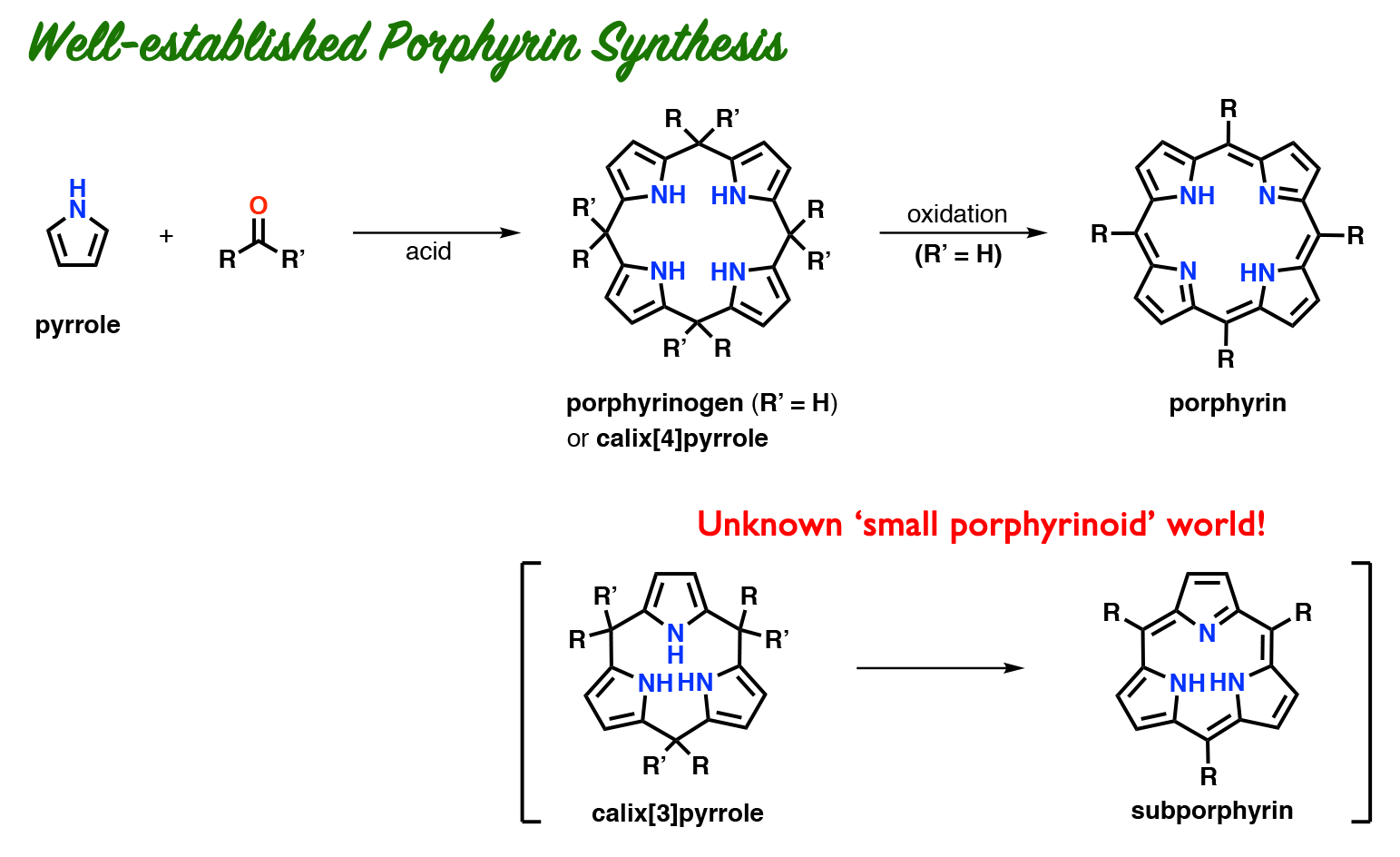
We have synthesized calix[3]pyrrole for the first time using a carbonyl rope as a precursor. Calix[3]pyrrole was found to be stable under neutral conditions. However, under standard porphyrin cyclization conditions (in the presence of acid), it underwent strain-induced ring expansion reaction within 10 seconds to give calix[6]pyrrole. On the other hand, boron-complex of calix[3]pyrrole tolerated acidic conditions, which explained the fact that contracted porphyrins with three pyrrole units, i.e. subporphyrin, were known as boron-complexed forms.
![calix[3]pyrrole synthesis](research/C3P.png)
Our findings have opened the door to a new ‘small porphyrinoids’ world!
Integrating Machine Learning and Organic Chemistry
We are also exploring new opportunities for organic chemistry research in the era of AI.
Machine learning (ML) is revolutionizing our lives, but the implementation of ML is still in its infancy. Based on our belief that ML will greatly help our future research, we are integrating ML with organic chemistry.
Creating 'Expert Eyes' with Image-based Machine Learning
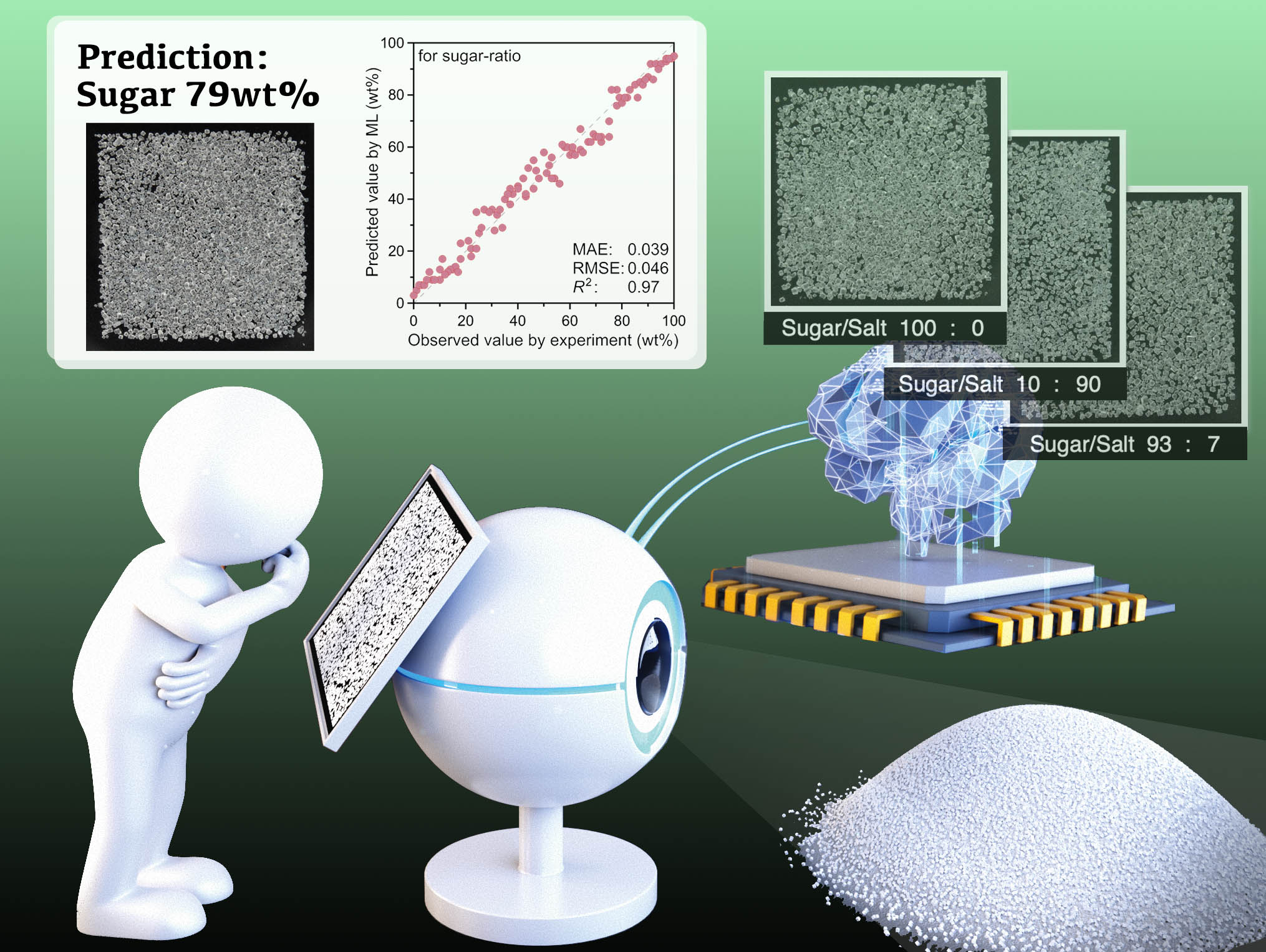
Our first effort with ML was to share the "experience and intuition" of researchers as ML models. As researchers gain research experience, they may be able to predict the composition or reaction yield of a mixture with some accuracy just by visual observation. Such an observational eye can be a powerful tool leading to new discoveries, but it is not easy to obtain for beginners.
Using image-based ML, we have created a system that can predict the mixing ratio of solid mixtures from a photograph. Our system can predict weight ratio of sugar/dietary salt, molar ratio of polymorphs, enantiomeric ratios from images of their mixtures with mean absolute error of 4~8%. ML enableed reproducible and repeatable prediction with accuracy better than the human eye. We are now applying this ML system to our organic chemistry research, such as Carbonyl Ropes and Calix[3]pyrrole analogues.
Chemistry of 'Carbonyl Ropes'
Our research on aliphatic polycarbonyl compounds, called 'carbonyl ropes', started with a dream of making magic ropes in chemistry.
In the biosynthesis of polyketides, various metabolites called polyketides are synthesized from polyketone chains as if they are magic ropes. Inspired by such beautiful biosynthesis, we designed an original ketone sequence of polyketone which has alternating 1,3-diketone and 1,4-diketone subunits. We are working on ‘magical synthesis’ of various functional organic compounds using the carbonyl ropes.
Elongation of carbonyl ropes
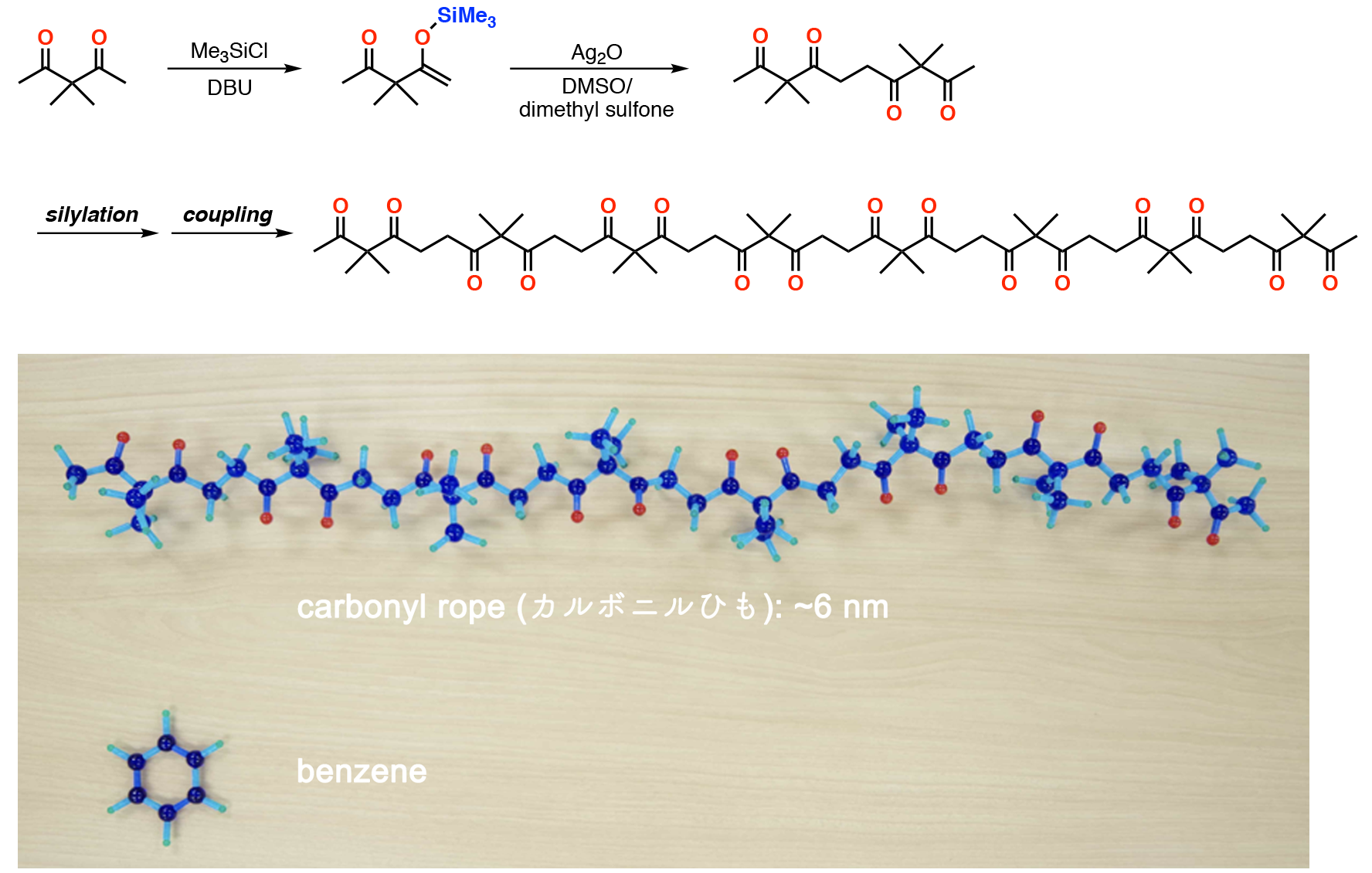
Our carbonyl ropes are synthesized by stepwise oligomerization of an acetylacetone derivatives. Namely, the chain length can be doubled by terminal ketone-selective silylation and subsequent oxidative homo-coupling reaction using silver(I) oxide. With this strategy, we have synthesized up to octameric acetylacetone chain with 6 nm length.
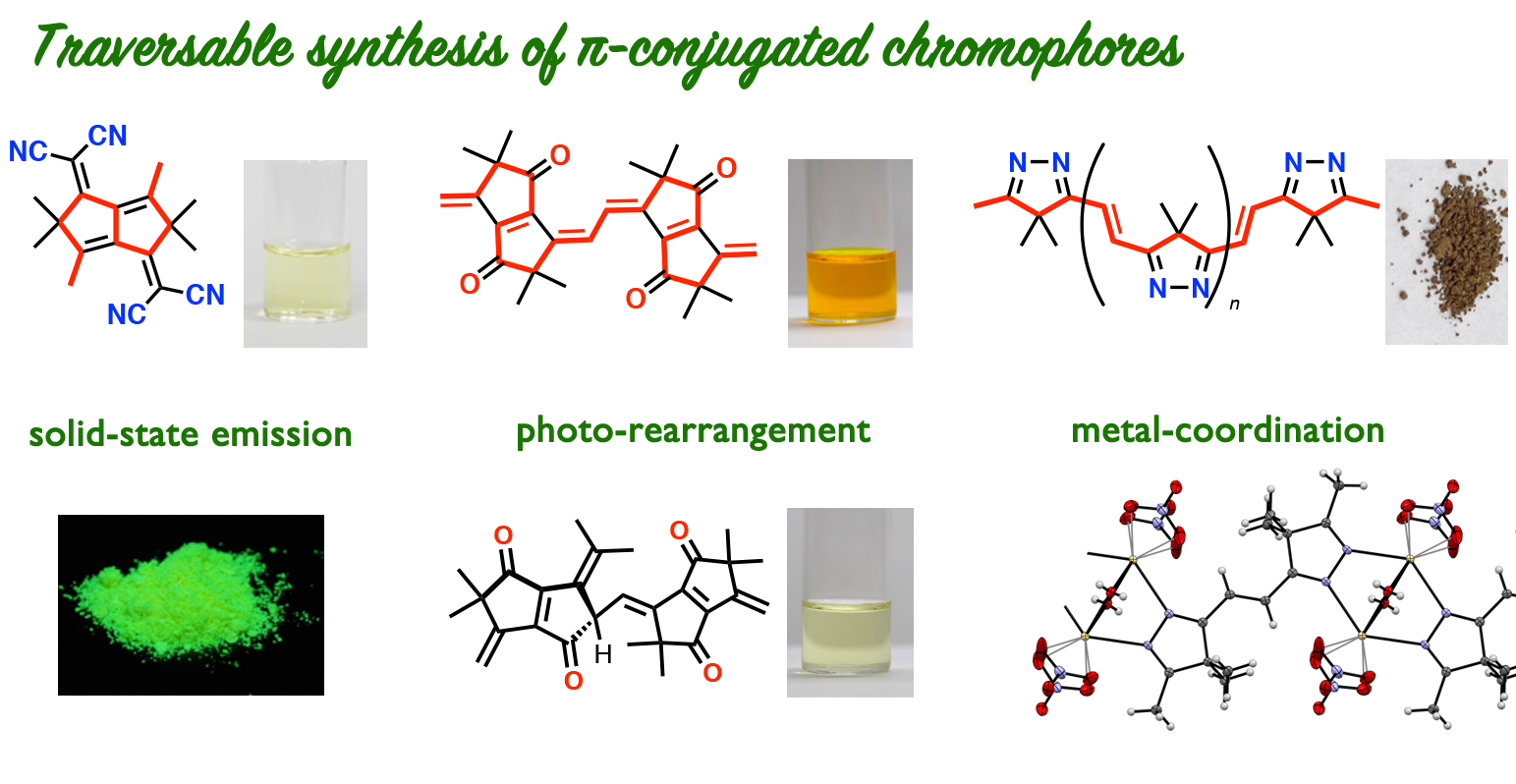
Traversable Synthesis of π–Conjugation
As natural polyketone chains are converted to fused six-membered π-conjugated system, our carbonyl rope can also be transformed to polycyclic compounds. Intramolecular condensation reaction of a short carbonyl rope gave pentalene-based, cross-conjugated system. After chemical modification at the remaining carbonyl groups, we have synthesized organic chromophores that emit visible light in the solid state.
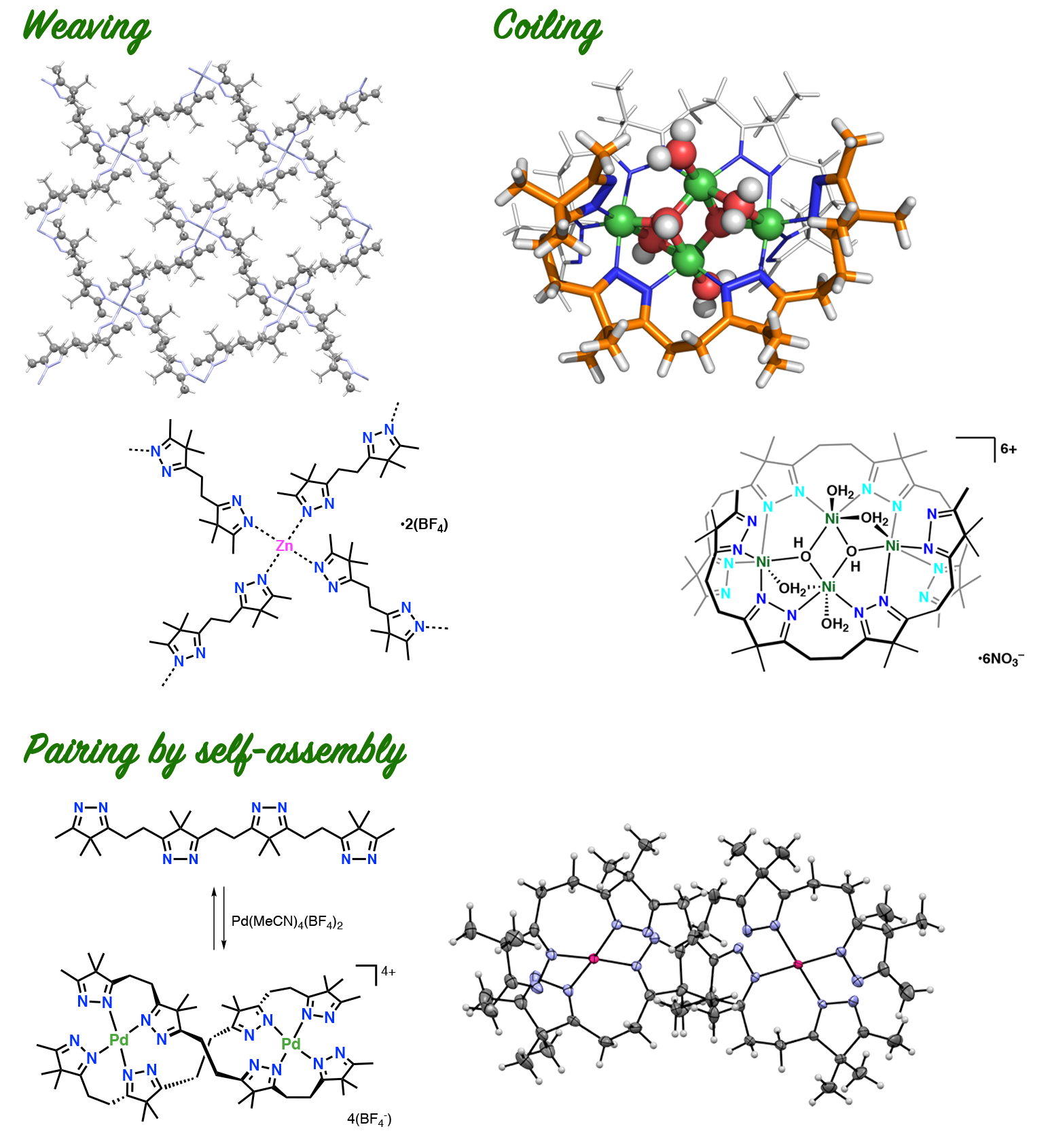
From Carbonyl Ropes to Imine Ropes: Weaving and Coiling
The carbonyl ropes were converted to imine ropes by treatment with hydrazine. The imine ropes can bind various metal ions to form assemblies. For example, complexation with Zn(II) ions resulted in the formation of textile-like coordination polymer as if imine chains were woven. When combined with Ni(II) ions, imine chains were coiled around a nickel oxide cluster. Self-assembly of imine chains occurs on complexation with Pd(II) ions.
References
For the details, please visit our publication page.
