論文リスト
| 94. | Interaction Force Landscapes within and Surrounding Hydrophobic Nanopockets Revealed by Three-Dimensional Scanning Atomic Force Microscopy M. Ogasawara, S. Tanaka, K. Komatsu, N. Kishimoto, D. Zhang, T. Yoneda, Y. Inokuma, M. Morimoto, A. Ohta, H. Asakawa J. Phys. Chem. C 2026, 130, 1004-1013. 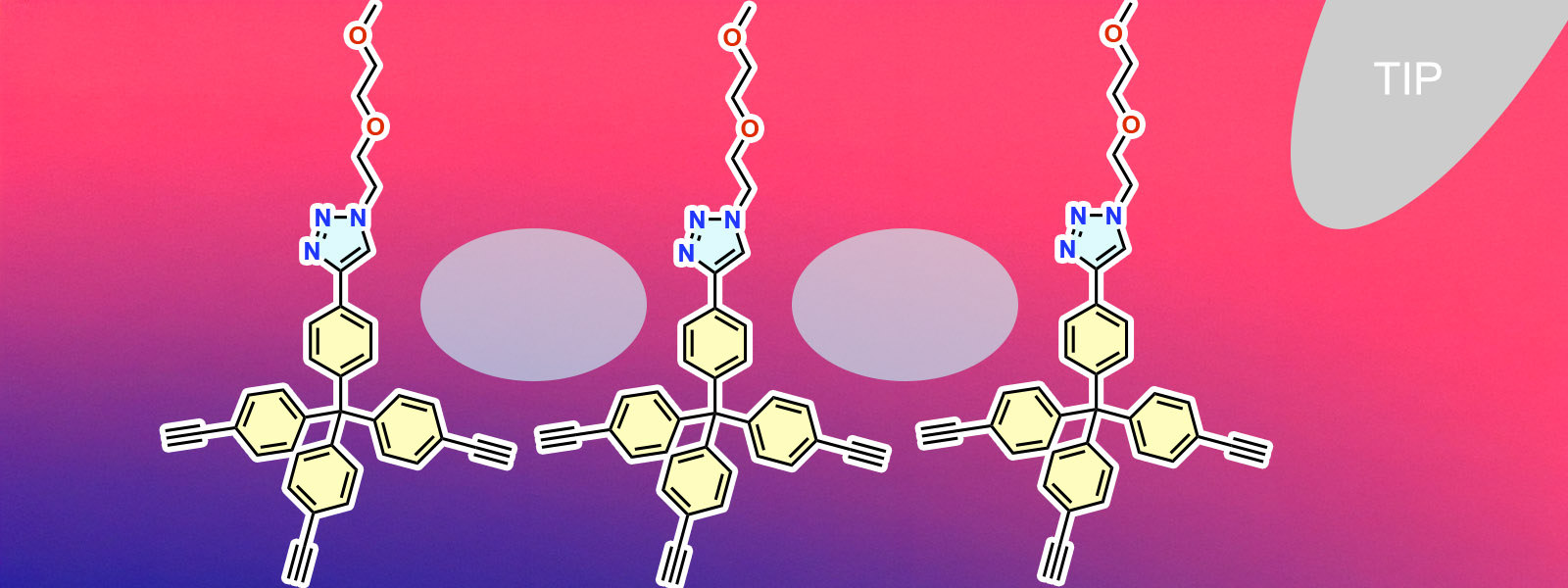 淺川先生(金沢大)との共同研究で、疎水性ナノポケットをAFMで可視化する研究を行いました。フロントカバーに採択されました! |
| 93. | Click Conjugation Using Discrete Polyketones Enables Dual Anchoring of Antibodies and Nanowires for Exosome Profiling K. Chattrairat, A. Yokoi, Y. Manabe, Y. Ide, T. Hasegawa, M. Iida, T. Ajiri, Z. Zhu, R. Uekusa, M. Kitagawa, Y. Baba, H. Kajiyama, Y. Inokuma, T. Yasui ChemRxiv DOI: 10.26434/chemrxiv-2025-n859x 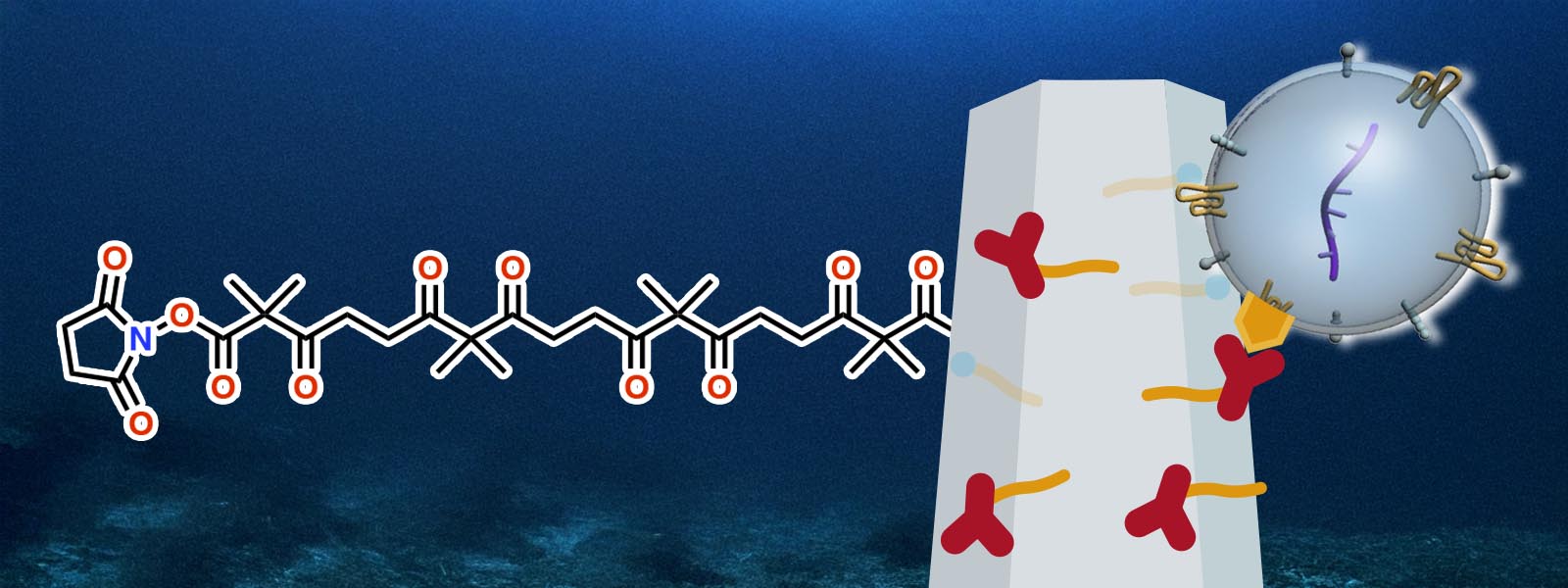 末端修飾ポリケトンを使ったZnOナノワイヤ表面の簡便な抗体修飾法を発見し、細胞外小胞の効率的な捕捉に繋げました。東京科学大の安井先生らとの共同研究です。 |
| 92. | Image-Based Machine Learning Using Inkjet-Printed Chemicals: Mixing Ratio Prediction and Metal Ion Detection T. Sano, Y. Terauchi, Y. Ide, I. Takigawa, T. Minami, Y. Inokuma Org. Lett. 2025, 27, 8841-8845. 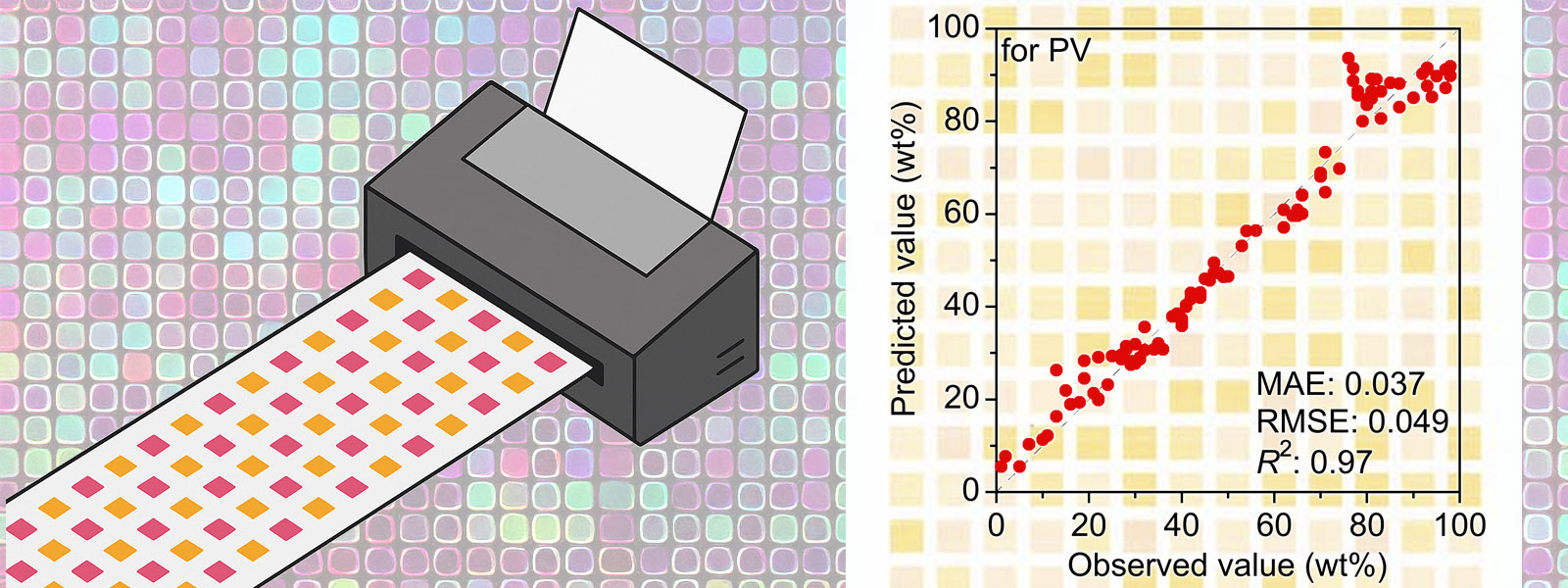 インクジェットプリンタで化合物を印刷することで、画像機械学習の教師データを効率的に取得するシステムを構築しました。東大の南先生、ICReDDの瀧川先生との共同研究です。フロントカバーにも採択されました! |
| 91. | Size-Selective Synthesis of Calix[n]furan[2n]thiazoles Bearing meso-CH2 Bridges K. Shibata, T. Nakabayashi, K. Watanabe, Y. Ide, T. Ichino, Y. Inokuma J. Porphyrins Phthalocyanines 2025, 29, 184–190. 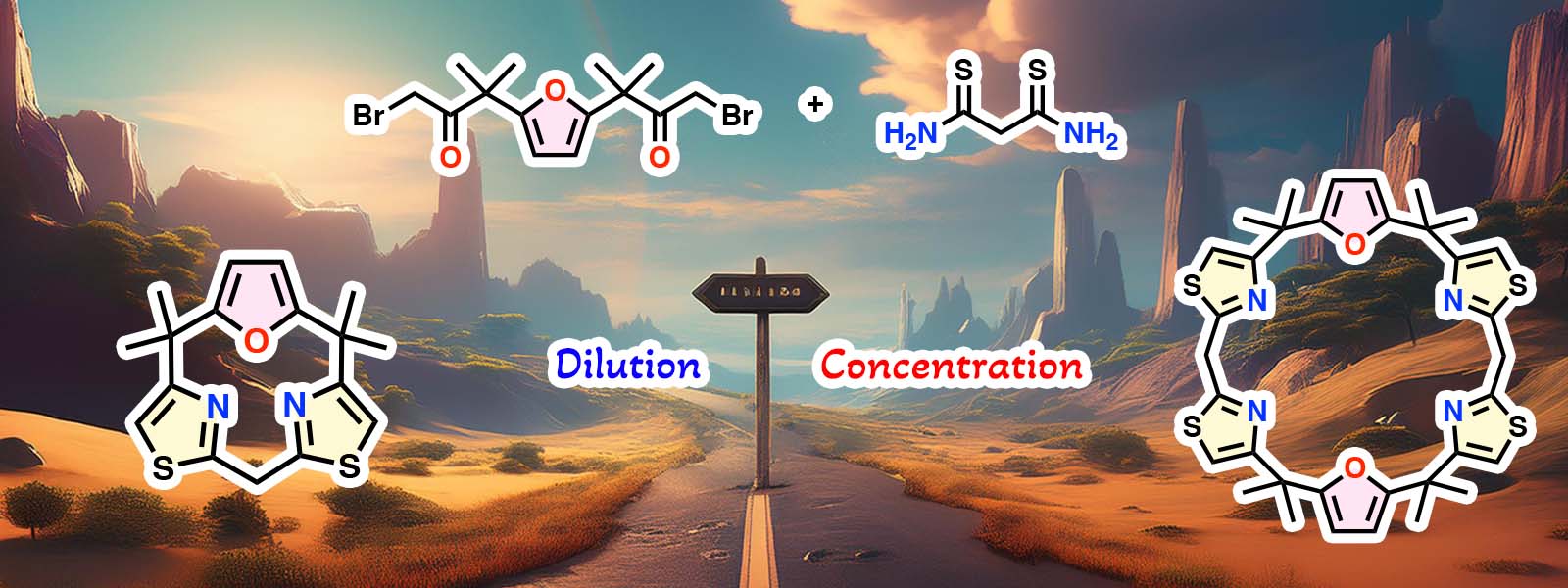 チアゾールを含むCalix[n]furan[2n]thiazolesの環サイズ選択的な合成を行いました。 Karl M. Kadish先生の80歳 記念特集号への招待投稿論文です。 |
| 90. | Cyclo[4]pyrrole with α–β Direct Linkages Y. Sun, R. Kitahara, T. Ichino, Y. Ide, H. Senboku, S. Shimizu, T. Tanaka, Y. Inokuma Chem. Sci. 2024, 15, 19571–19576. 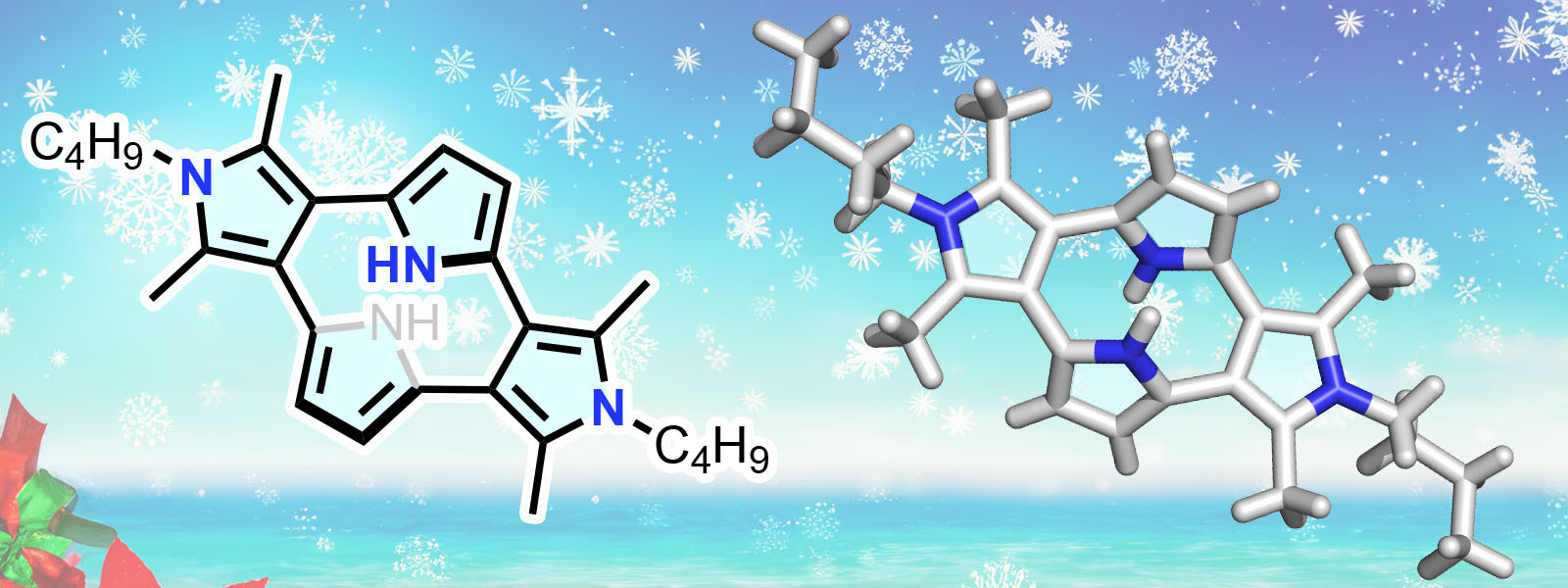 ポリケトンを用いたcyclo[4]pyrroleの合成に成功し、電気化学・分光学・計算化学によって構造・物性を明らかにしました。九大・清水宗治先生、京大・田中隆行先生、ICReDD・市野博士との共同研究成果です。 |
| 89. | Hydride Content Control of Perovskite Oxyhydride BaTiO3–xHx Supported by Image-Based Machine Learning T. Sano, Y. Ide, T. Tsumori, H. Ubukata, I. Takigawa, H. Kageyama, Y. Inokuma ACS Appl. Eng. Mat. 2024, 2, 2391–2396.  画像機械学習を使って酸水素化物中のヒドリド量を精度高く予測・制御することに成功しました。ICReDD・瀧川先生、京大・陰山先生との共同研究成果です。 |
| 88. | A Molecular Dynamics-Based Conformational Simulation Method for Analysis of Arrival Time Distributions in Ion Mobility Mass Spectrometry K. Tashiro, Y. Ide, T. Taketsugu, K. Ohara, K. Yamaguchi, M. Kobayashi, Y. Inokuma Adv. Theory Simul. 2024, 7, 202400691. 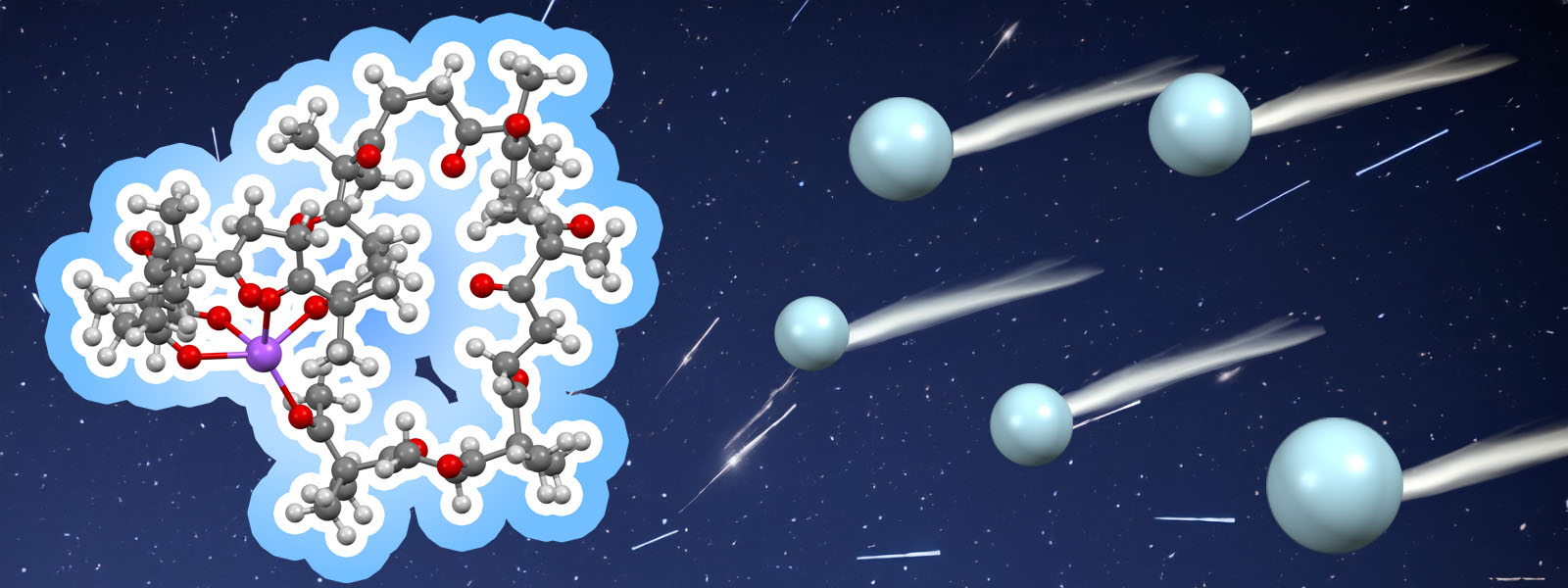 イオンモビリティー質量分析のための衝突断面積シミュレーション法をポリケトンの実測データを基に開発しました。小林先生、武次先生、小原先生、山口先生との共同研究成果です。 |
| 87. | Discrete Polyketones: Synthesis, Derivatization, and Potential Applications Y. Inokuma Bull. Chem. Soc. Jpn. 2024, 97, uoae072. 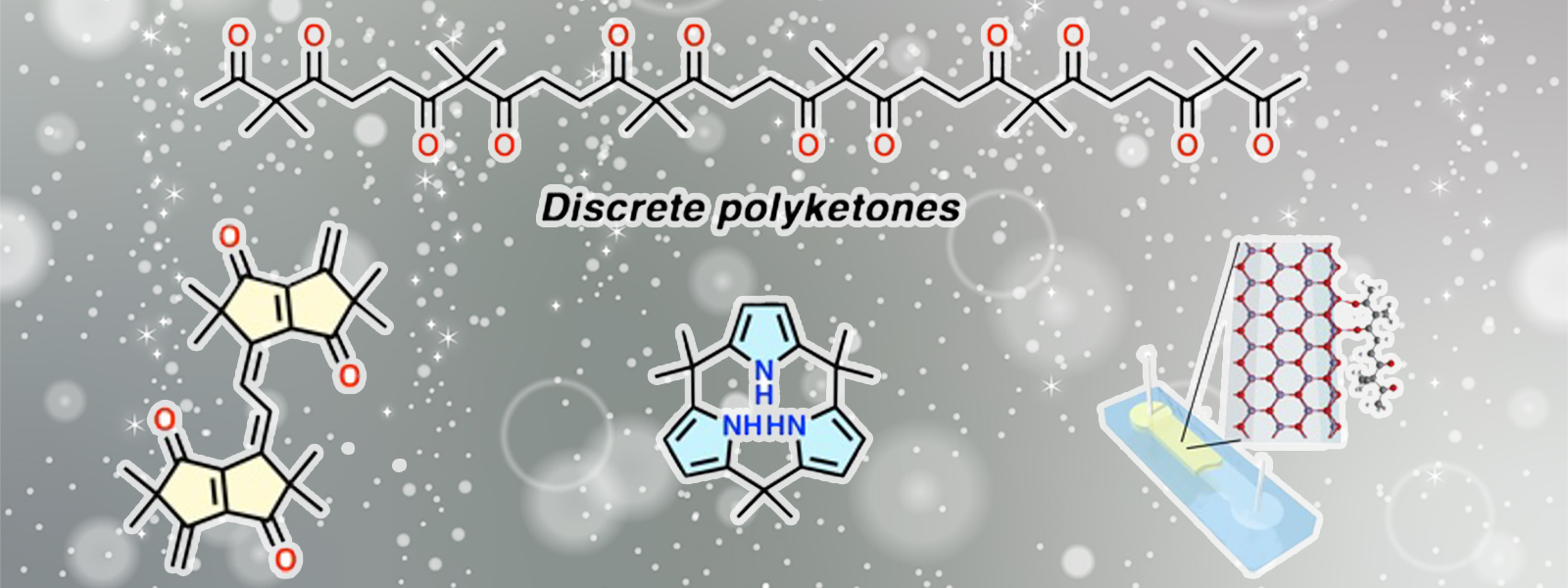 単分散ポリケトンを基盤とする類縁体の機能性分子への応用例をまとめました。 日本化学会第41回学術賞の受賞レビューです。 |
| 86. | Contracted Porphyrins and Calixpyrroles: Synthetic Challenges and Ring-Contraction Effects K. Watanabe, N. N. Pati, Y. Inokuma Chem. Sci. 2024, 15, 6994–7009. 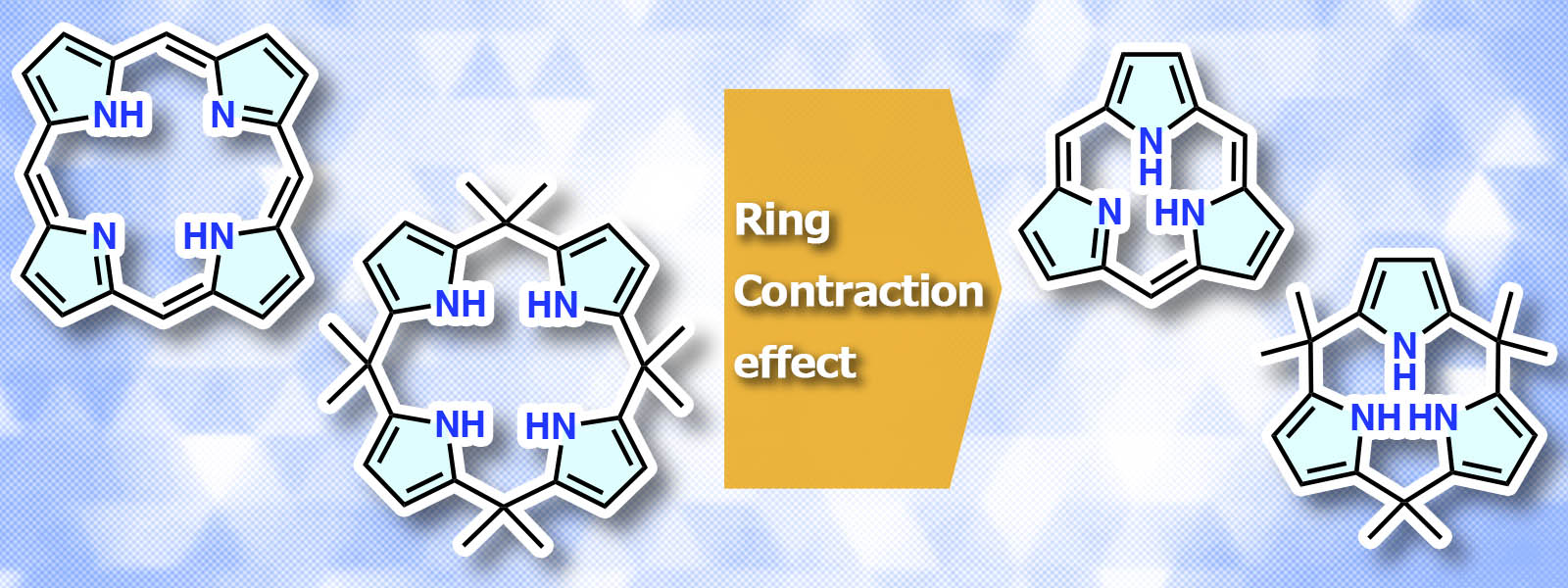 3つのピロール環で構成されるポルフィリン類縁体の特異な環縮小効果について報告しました。 |
| 85. | Implementation of Highly Crystalline Polyketones as Solid Polymer Electrolytes in high-temperature Lithium Metal Batteries R. Andersson, I. L Johansson, K. I. Shivakumar, G. Hernández, Y. Inokuma, J. Mindemark Solid State Ionics 2024, 410, 116542. 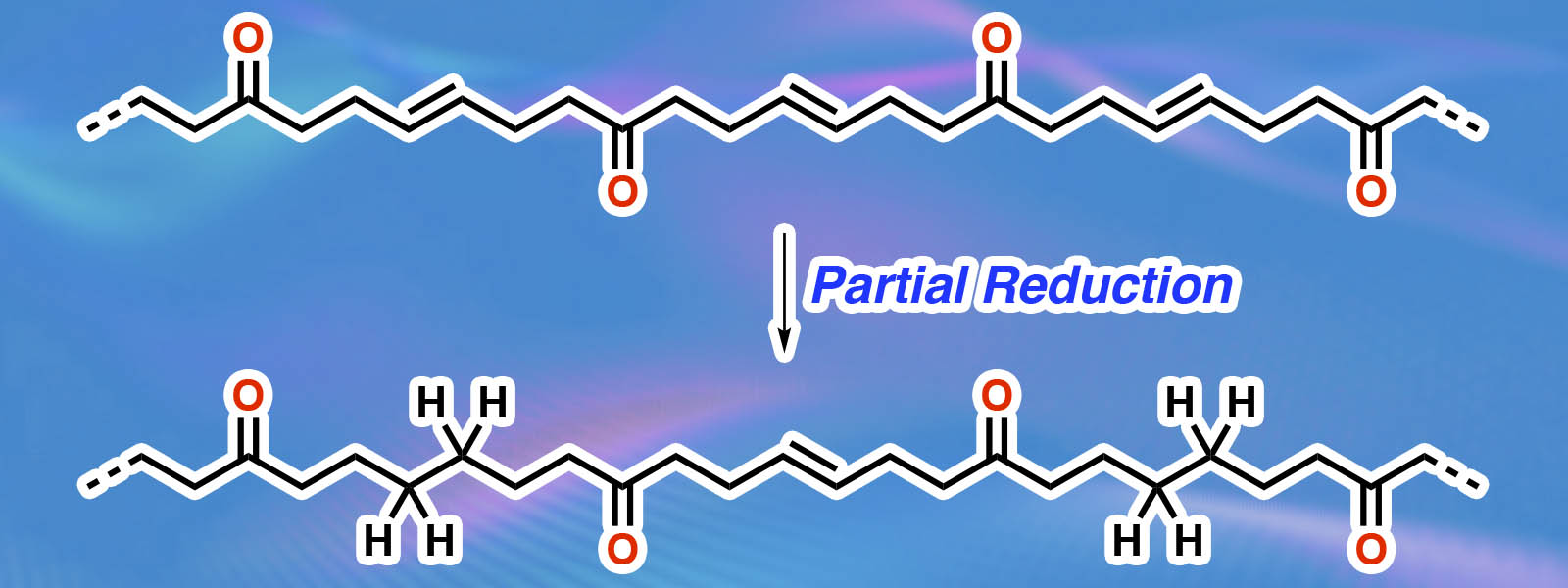 ポリケトンの固体高分子電解質への応用論文の第3弾。ICReDD MANABIYA(ACADEMIC)の共同研究成果です。 |
| 84. | One-pot, Gram-Scale Synthesis of Calix[5]- and Calix[6]furan: Derivatization for Polyketone- and Isopyrazole-based Macrocycles with Conformational Analysis T. Yoneda, T. Sano, N. N. Pati, Y. Ide, Y. Inokuma Asian J. Org. Chem. 2024, 13, e202400023. 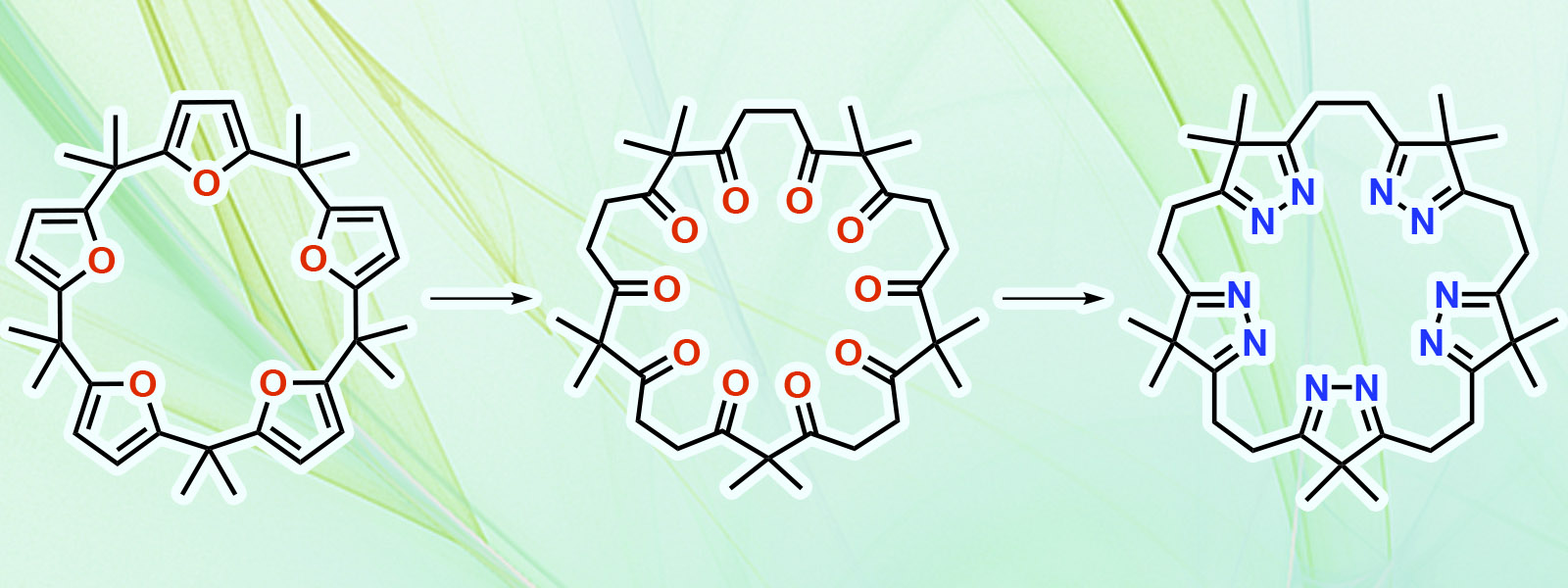 Calix[n]furan (n = 5, 6) のグラムスケール合成法を開発し、環状ポリケトンとイソピラゾールへと変換しました。 |
| 83. | Strain-Based Design, Direct Macrocyclization, and Metal Complexation of Thiazole-Containing Calix[3]pyrrole Analogues K. Watanabe, K. Shibata, T. Ichino, Y. Ide, T. Yoneda, S. Maeda, Y. Inokuma Inorg. Chem. Front. 2024, 11, 3548–3554. ChemRxiv DOI: 10.26434/chemrxiv-2024-6nqnq 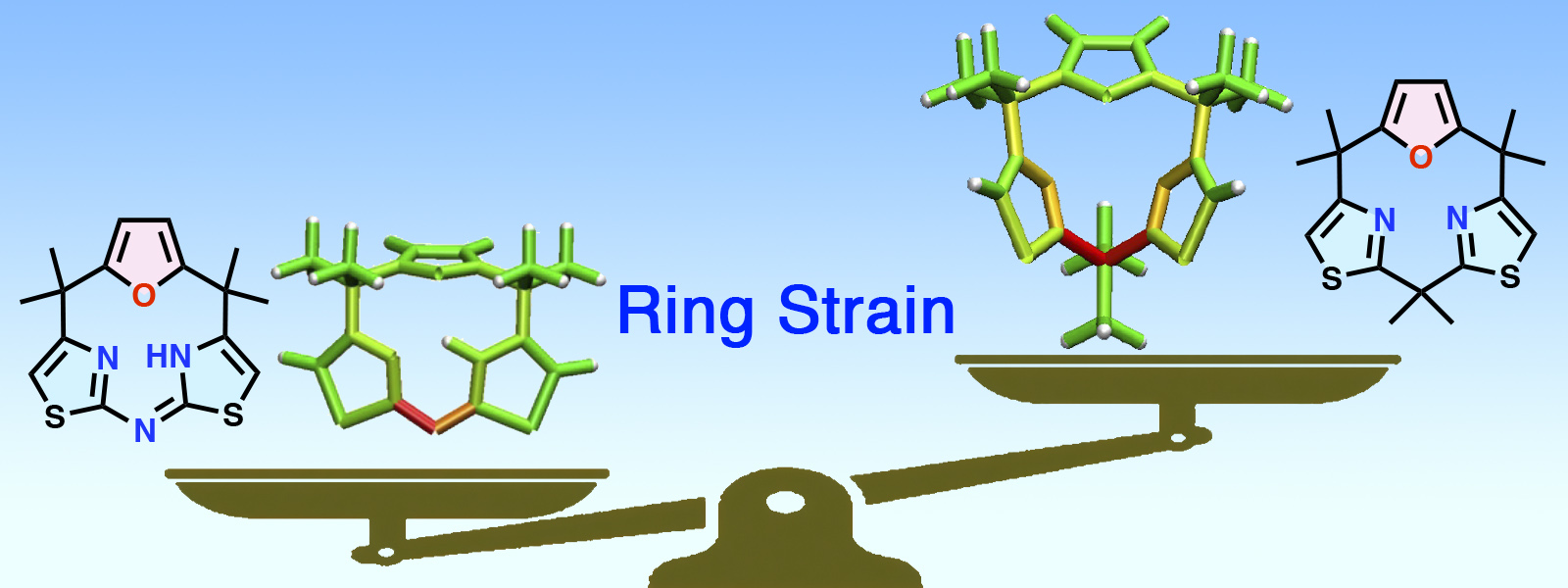 歪みエネルギーの計算と可視化によって直接環化合成可能なCalix[3]pyrrole誘導体をデザインし、錯体化学へと繋げました。前田グループとの共同研究です。 |
| 82. | Drug–drug Conjugates of MEK and Akt Inhibitors for RAS-mutant Cancers H. Fujita, S. Arai, H. Arakawa, K. Hamamoto, T. Kato, T. Arai, N. Nitta, K. Hotta, N. Hosokawa, T. Ohbayashi, C. Takahashi, Y. Inokuma, I. Tamai, S. Yano, M. Kunishima, Y. Watanabe Bioorg. Med. Chem. 2024, 102, 117674. ChemRxiv DOI: 10.26434/chemrxiv-2023-ps4h1-v2 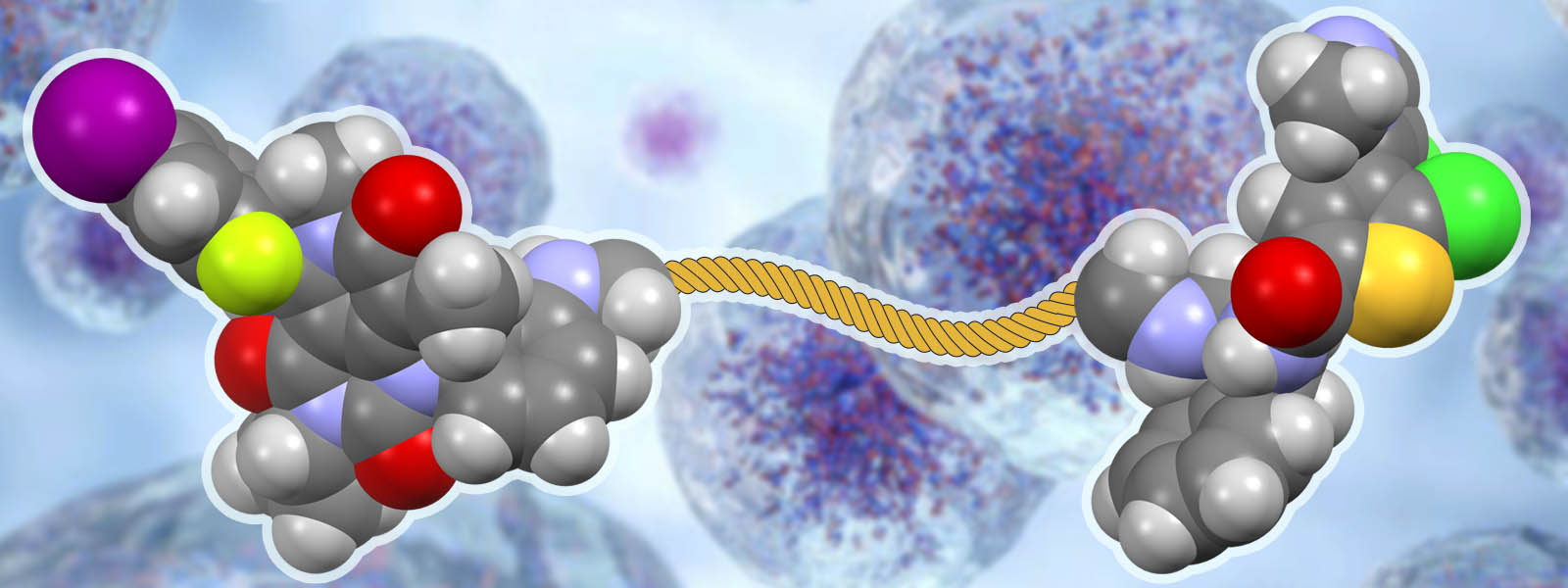 金沢大医学部の渡部先生、藤田先生らとの共同研究において、カルボニルひもから誘導されるリンカーを使ってMEKとAktの両経路に作用する複合抗がん薬を開発しました。 |
| 81. | Machine Learning-Based Analysis of Molar and Enantiomeric Ratios and Reaction Yields Using Images of Solid Mixtures Y. Ide, H. Shirakura, T. Sano, M. Murugavel, Y. Inaba, S. Hu, I. Takigawa, Y. Inokuma Ind. Eng. Chem. Res. 2023, 62, 13790-13798. ChemRxiv DOI: 10.26434/chemrxiv-2023-3gdsb 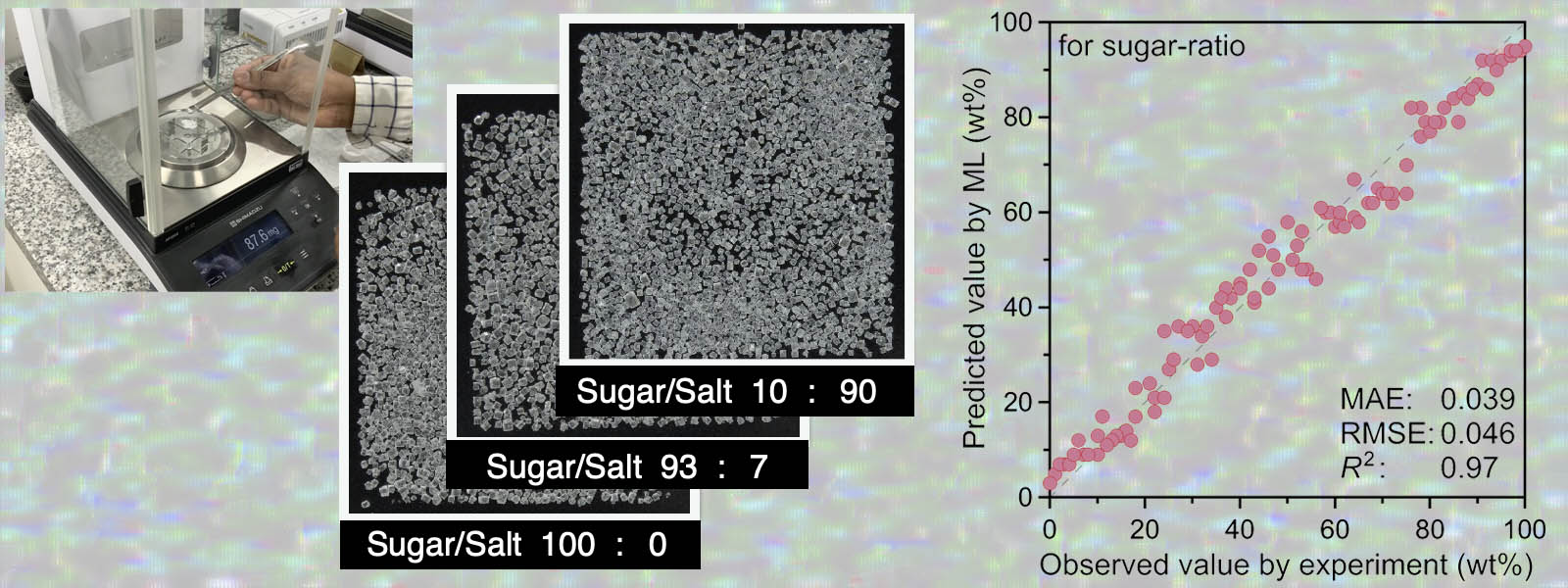 固体混合物の写真をもとに機械学習でモル比、鏡像体比、結晶多形の比率、反応収率を求めるシステムを開発しました。 カバーピクチャにも採択されました! プレスリリースは、こちら。 本研究の記事が日本経済新聞に掲載されました。 本研究の記事が産経新聞およびiza(イザ!)に掲載されました。 本研究の記事が朝日新聞デジタルに掲載されました。 |
| 80. | Identifying High-Grade Serous Ovarian Carcinoma-Specific Extracellular Vesicles by Polyketone-coated Nanowires A. Yokoi, M. Ukai, T. Yasui, Y. Inokuma, K. Hyeon-Deuk, J. Matsuzaki, K. Yoshida, M. Kitagawa, K. Chattrairat, M. Iida, T. Shimada, Y. Manabe, I-Y. Chang, E. Asano-Inami, Y. Koya, A. Nawa, K. Nakamura, T. Kiyono, T. Kato, A. Hirakawa, Y. Yoshioka, T. Ochiya, T. Hasegawa, Y. Baba, Y. Yamamoto, H. Kajiyama Sci. Adv. 2023, 9, eade6958 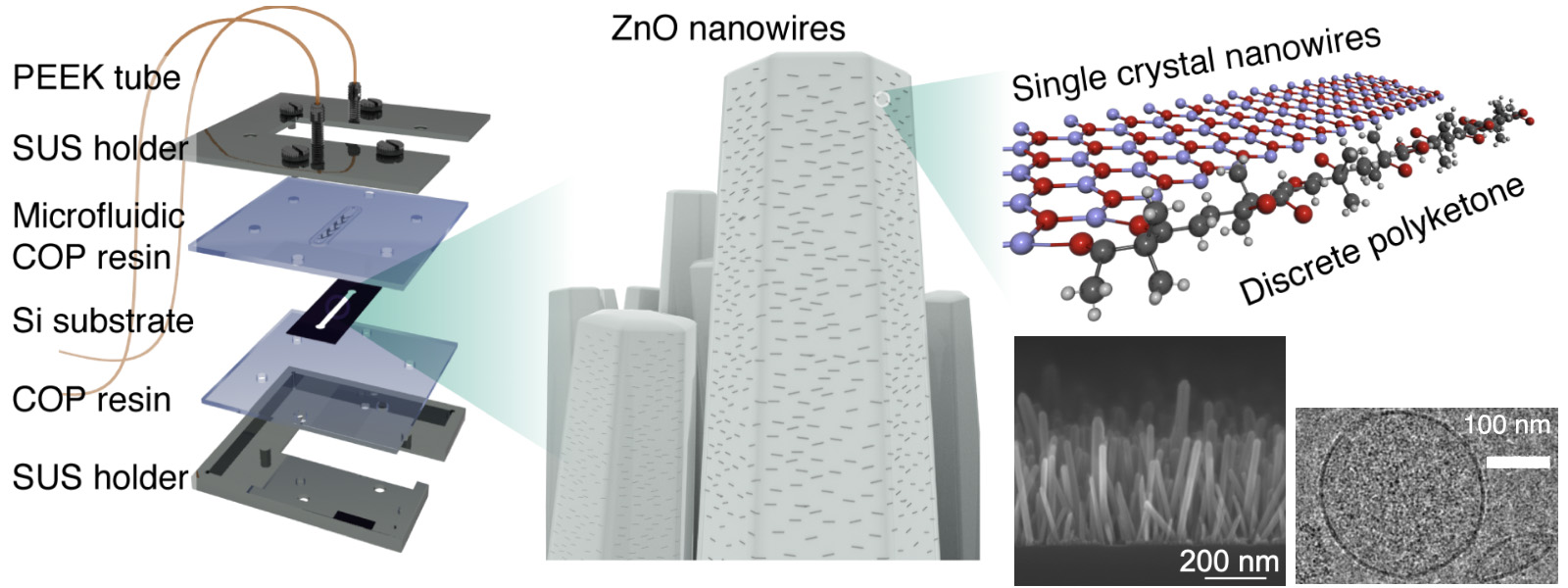 我々のポリケトンをコーティングしたZnOナノワイヤで卵巣がんの検出が可能な細胞外小胞の捕捉に成功しました。 名大 横井先生、東工大 安井先生、京大 金先生らとの共同研究成果です。 JSTのプレスリリースは、こちら。 |
| 79. | The Geometry of Calix[3]pyrrole and the Formation of the Calix[3]pyrrole·F–Complex in Solution R. Saha, J. Pirillo, Y. Ide, Y. Inokuma, Y. Hijikata Theor. Chem. Acc. 2023, 142, Article Number 50. 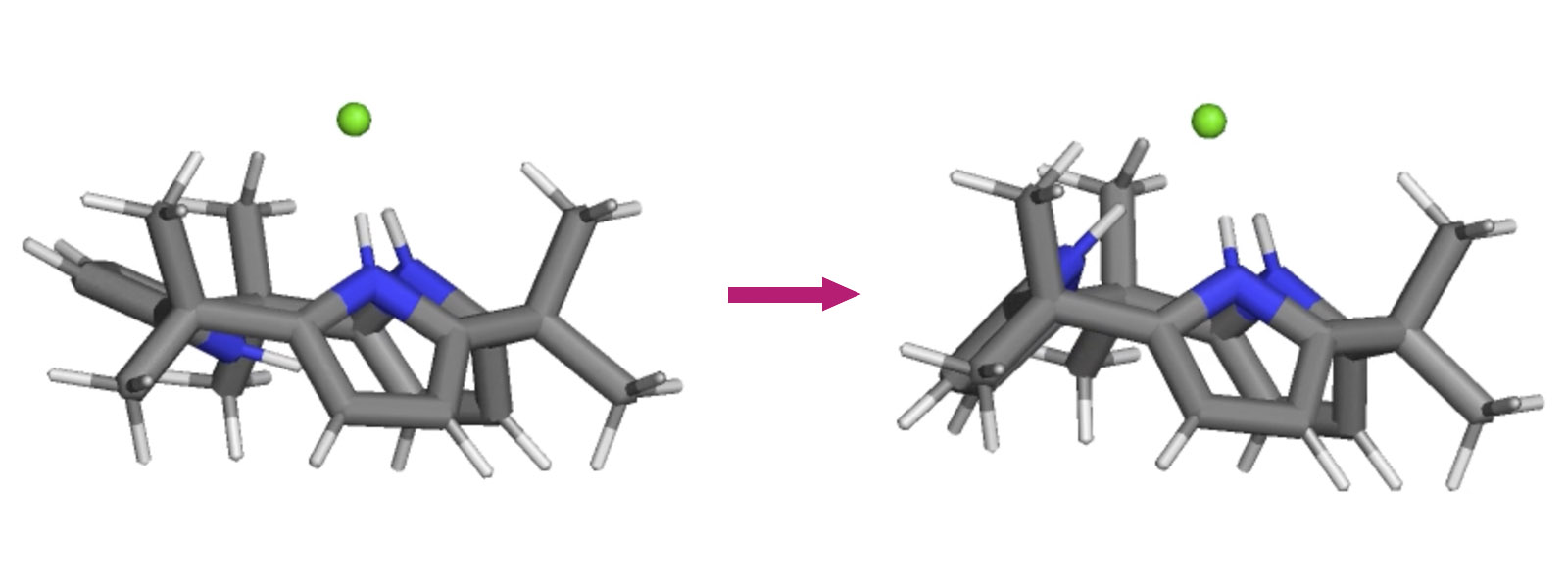 Calix[3]pyrroleがフッ化物イオンと結合する際の構造解析をMDを使って行いました。土方グループとの共同研究です。 |
| 78. | Chain Length-Dependent Hydrogen-Bonded Self-Assembly of Terminally Functionalized Discrete Polyketones K. I. Shivakumar, Y. Manabe, T. Yoneda, Y. Ide, Y. Inokuma Precis. Chem. 2023, 1, 34-39. 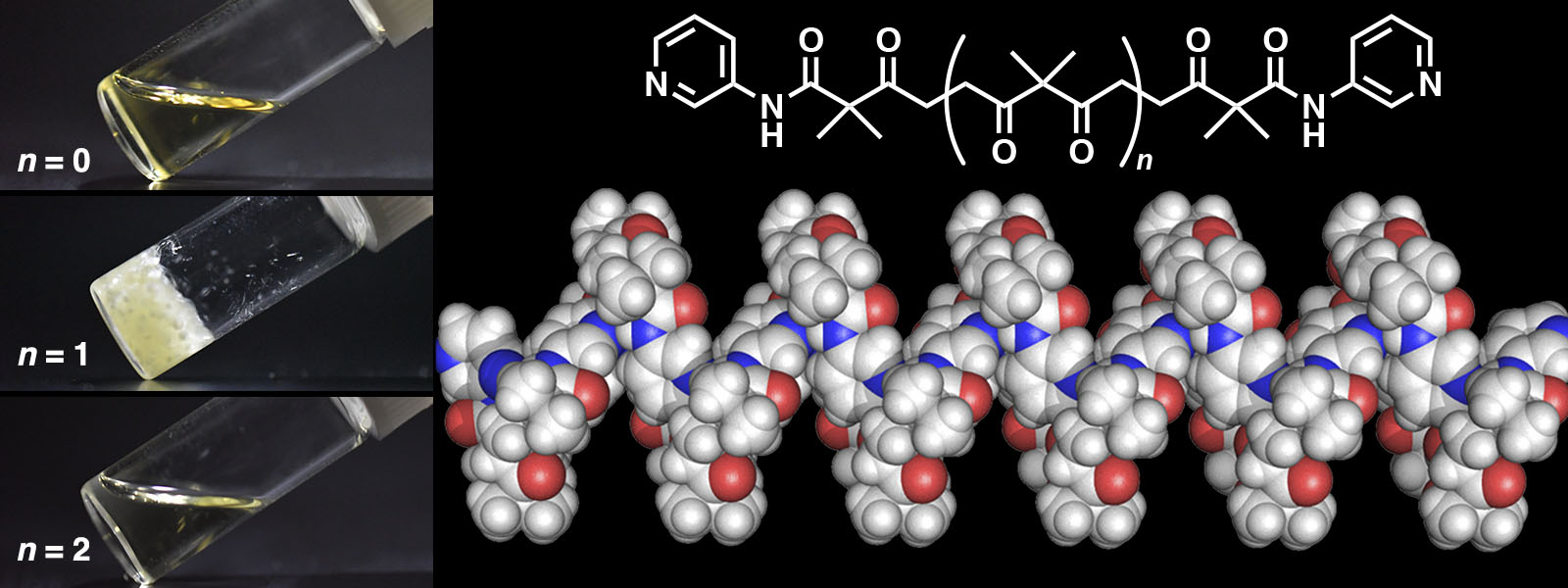 末端に水素結合部位を持つポリケトンの自己集合構造を解析しました。 |
| 77. | Chiral Calix[3]pyrrole Derivatives: Synthesis, Racemization Kinetics, and Ring Expansion to Calix[9]- and Calix[12]pyrrole Analogues Y. Inaba, J. Yang, Y. Kakibayashi, T. Yoneda, Y. Ide, Y. Hijikata, J. Pirillo, R. Saha, J. L. Sessler, Y. Inokuma Angew. Chem. Int. Ed. 2023, 62, e202301460 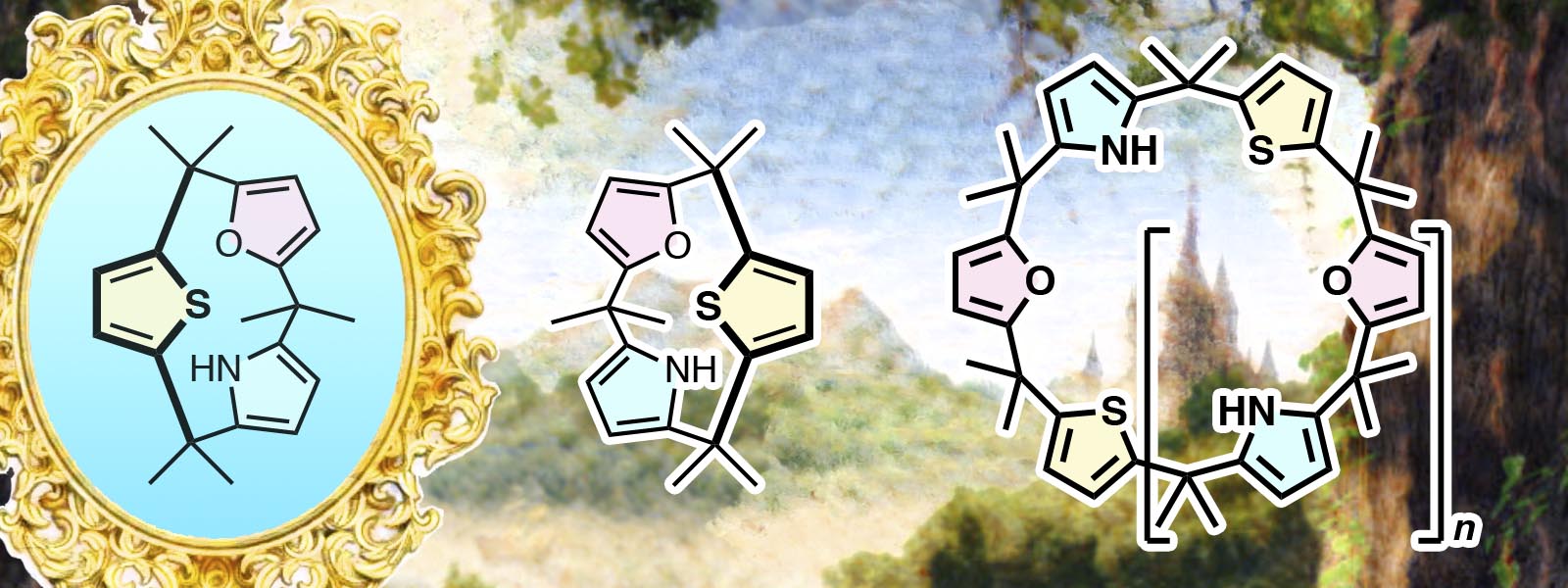 キラルCalix[3]pyrrole類縁体の合成と歪み誘起環拡大反応によって、calix[12]タイプの大環状化合物を合成しました。類縁体の結晶構造としては世界最大のものを決定しました。 |
| 76. | Toward Calix[2]-Type Macrocycles: Synthesis and Structural Analysis of Cyclic Tetraketone and Highly Strained Furanophane T. Sano, Y. Sun, T. Mukai, Y. Inaba, T. Yoneda, Y. Ide, J. Pirillo, Y. Hijikata, Y. Inokuma J. Porphyrins Phthalocyanines 2023, 27 , 1067-1073. 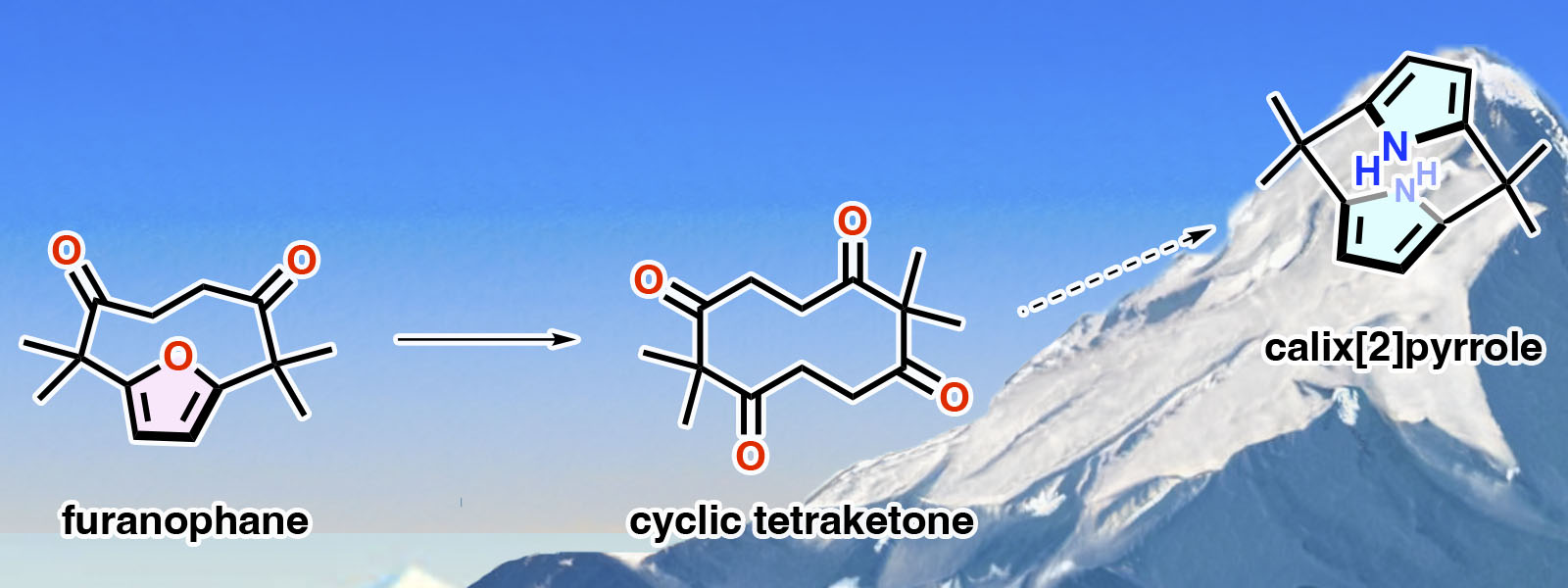 Calix[2]pyrrole合成に向けた新たな挑戦の第一歩です。 Jonathan L. Sessler先生の65歳 記念特集号への招待投稿論文です。 |
| 75. | Absorption Spectra of Calix[3]pyrrole Analogues as Probes for Contracted Macrocycles K. Watanabe, R. Saha, Y. Inaba, Y. Manabe, T. Yoneda, Y. Ide, Y. Hijikata, Y. Inokuma J. Porphyrins Phthalocyanines 2023, 27, 157-163. 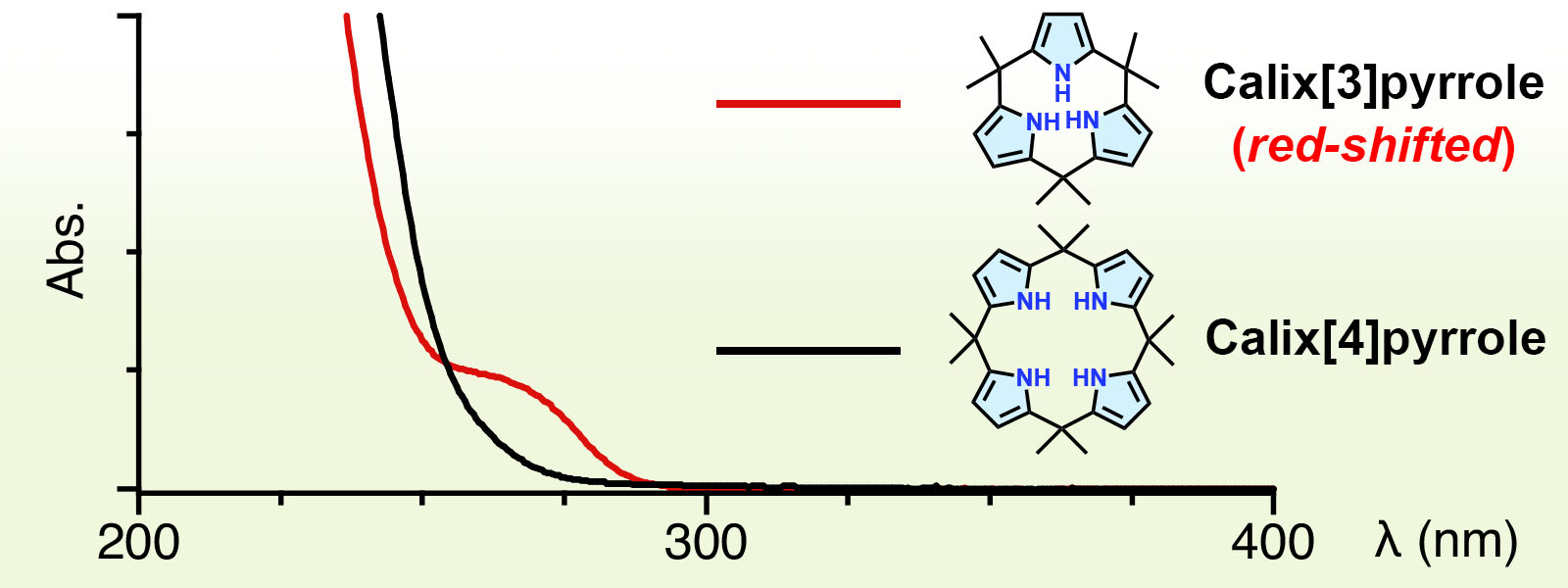 Calix[3]pyrroleに特徴的な紫外吸収スペクトルの長波長シフトを見つけました。 Tomás Torres先生の70歳 記念特集号への招待投稿論文です。 |
| 74. | Carbonyl-Containing Solid Polymer Electrolyte Host Materials: Conduction and Coordination in Polyketone, Polyester and Polycarbonate Systems T. Eriksson, H. Gudla, Y. Manabe, T. Yoneda, D. Friesen, C. Zhang, Y. Inokuma, D. Brandell, J. Mindemark Macromolecules 2022, 55, 10940-10949.  スウェーデン・ウプサラ大 Jonas Mindemark先生との国際共著第2弾です。 |
| 73. | Determination of the Critical Chain Length for Macromolecular Crystallization Using Structurally Flexible Polyketones Y. Ide, Y. Manabe, Y. Inaba, Y. Kinoshita, J. Pirillo, Y. Hijikata, T. Yoneda, K. I. Shivakumar, S. Tanaka, H. Asakawa, Y. Inokuma Chem. Sci. 2022, 13, 9848–9854. 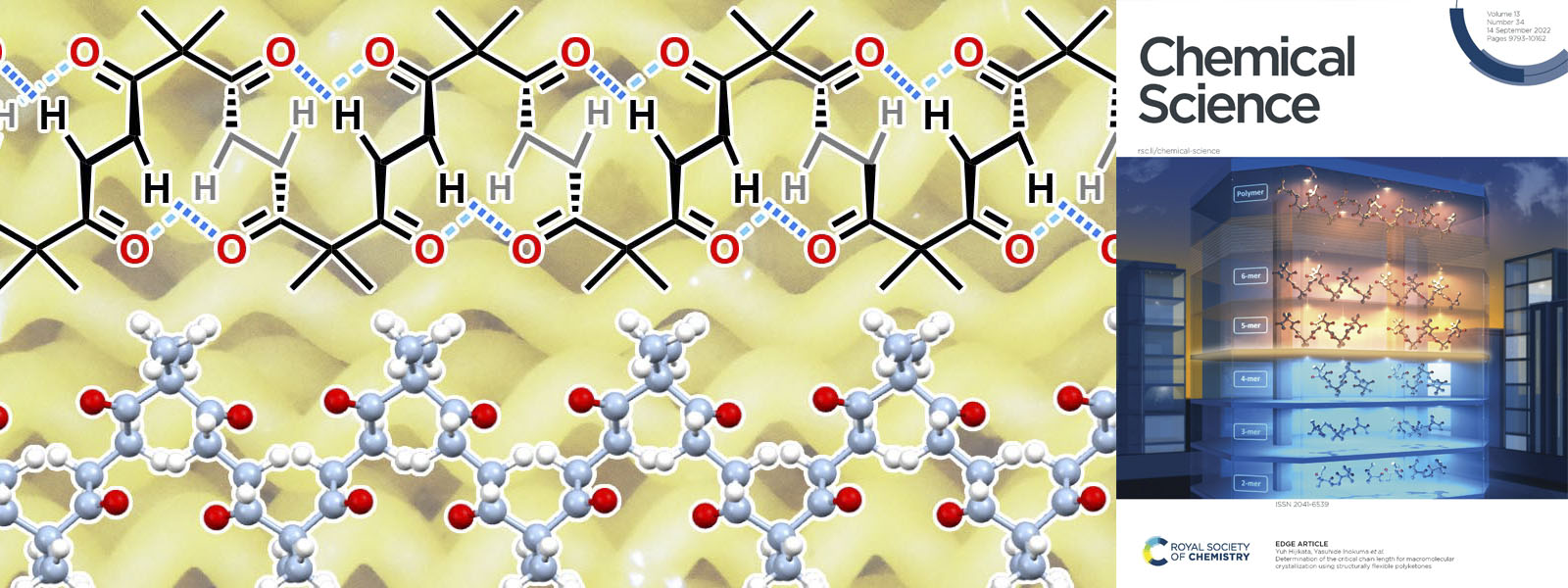 カルボニルひもをだんだん長くしていくと、ある長さで結晶構造が劇的に変化することを明らかにしました。 ICReDDの土方先生、金沢大学の浅川先生との共著論文です。 Front Coverに採択されました! |
| 72. | Alkali Metal Ion Binding Using Cyclic Polyketones N. Ozawa, K. I. Shivakumar, M. Murugavel, Y. Inaba, T. Yoneda, Y. Ide, J. Pirillo, Y. Hijikata, Y. Inokuma Chem. Commun. 2022, 58, 2971–2974. 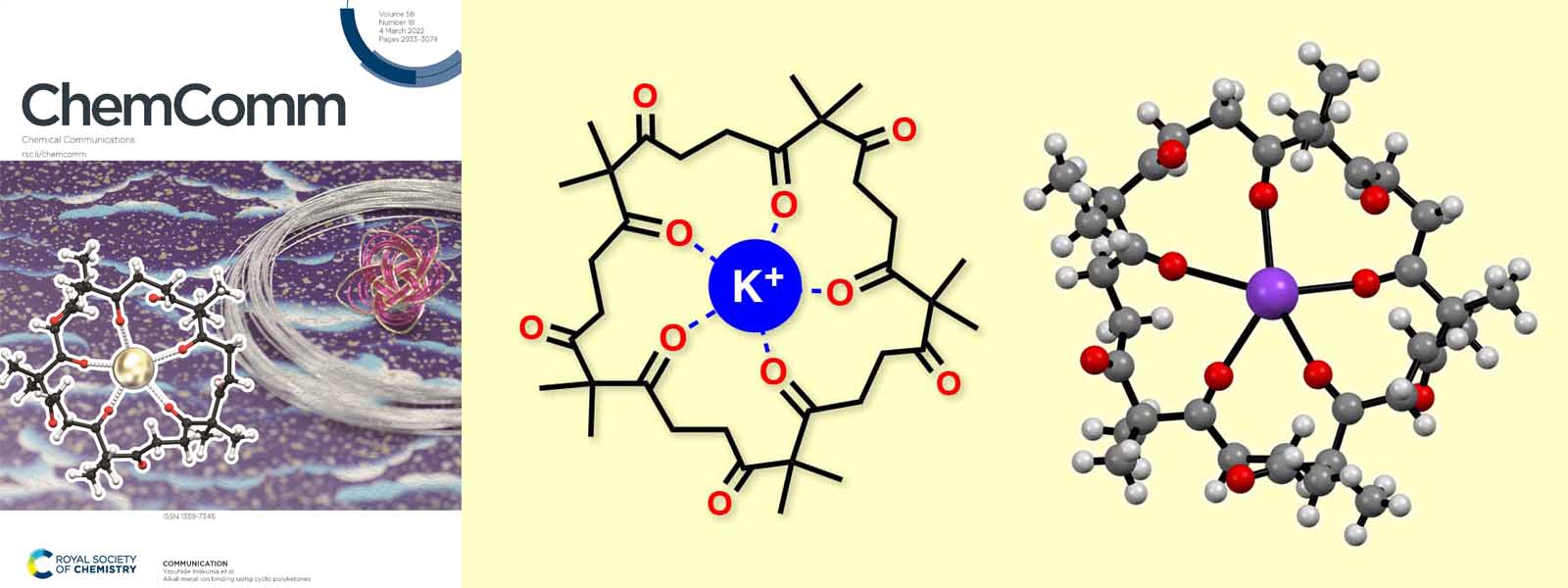 カルボニルひもの輪がアルカリ金属イオンを包接することを明らかにしました。 Chemical Communications HOT Articles 2022に選出されました! Inside Front Coverに選出されました! |
| 71. | Strain-induced Ring Expansion Reactions of Calix[3]pyrrole-related Macrocycles Y. Inaba, Y. Kakibayashi, Y. Ide, J. Pirillo, Y. Hijikata, T. Yoneda, Y. Inokuma Chem. Eur. J. 2022, 28, e202200056.  Calix[3]pyrroleの類縁体を合成して、環拡大反応の適用範囲、反応機構を明らかにしました。 |
| 70. | Hybrid Eu(III) Coordination luminophore Standing on Silica Nanoparticles by Two Legs for Enhanced Luminescence T. Zhang, Y. Kitagawa, R. Moriake, P. P. F. Rosa, M. J. Islam, T. Yoneda, Y. Inokuma, K. Fushimi, Y. Hasegawa Chem. Eur. J. 2021, 27, 14438–14443. 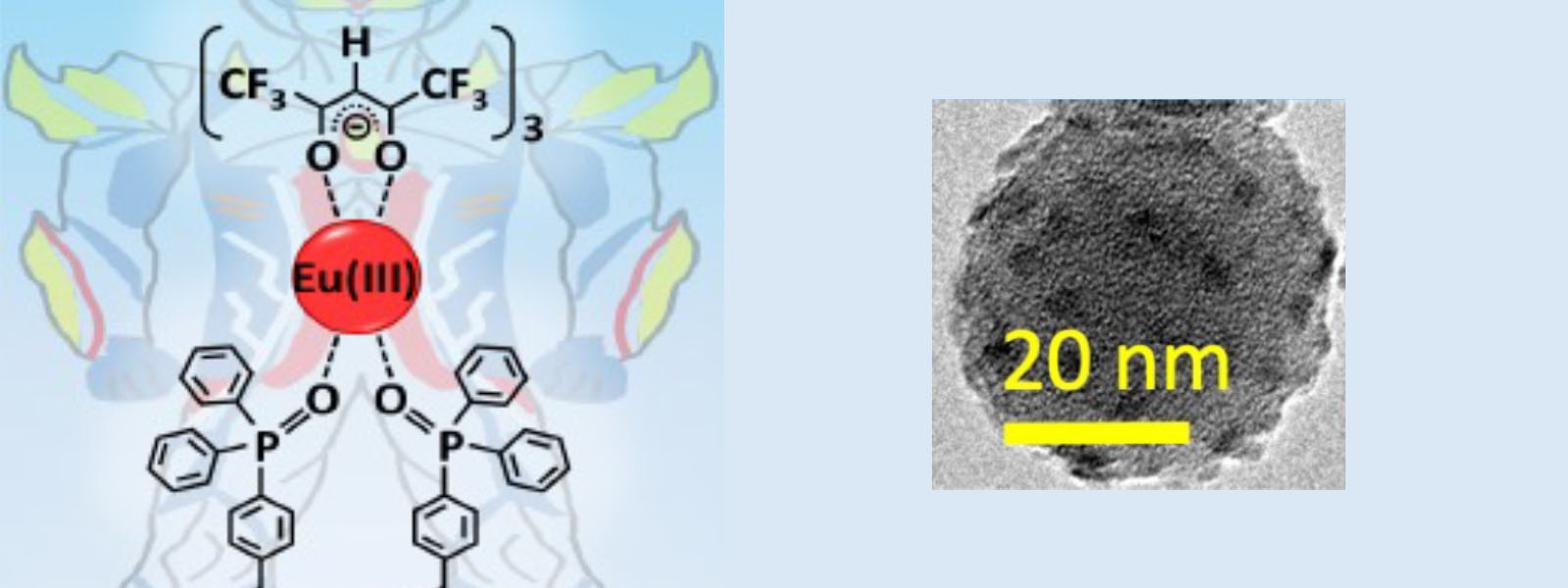 同学科の長谷川先生との共著論文です。配位子の合成をお手伝いさせて頂きました。 |
| 69. | Calix[3]pyrrole: A Missing Link in Porphyrin-Related Chemistry Y. Inaba, Y. Nomata, Y. Ide, J. Pirillo, Y. Hijikata, T. Yoneda, A. Osuka, J. L. Sessler, Y. Inokuma J. Am. Chem. Soc. 2021, 143, 12355-12360. 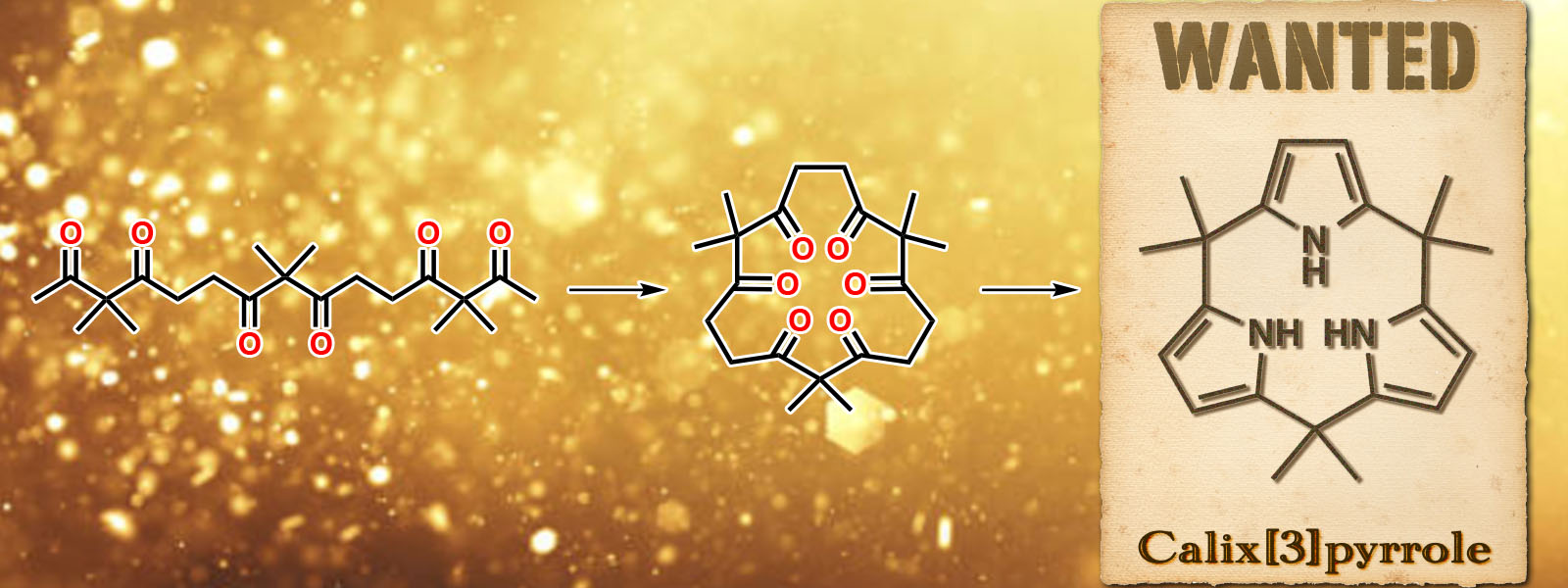 14年越しの夢、ついに叶う! カルボニルひもからcalix[3]pyrroleの合成に成功。 Science誌の"In Other Journals"にてハイライトされました。 Synfactsにてハイライトされました。 プレスリリースは、こちら。 |
| 68. | Isopyrazole-Masked Tetraketone: Tautomerism and Functionalization for Fluorescent Metal Ligands H. Shirakura, Y. Manabe, C. Kasai, Y. Inaba, M. Tsurui, Y. Kitagawa, Y. Hasegawa, T. Yoneda, Y. Ide, Y. Inokuma Eur. J. Org. Chem. 2021, 4345–4349. 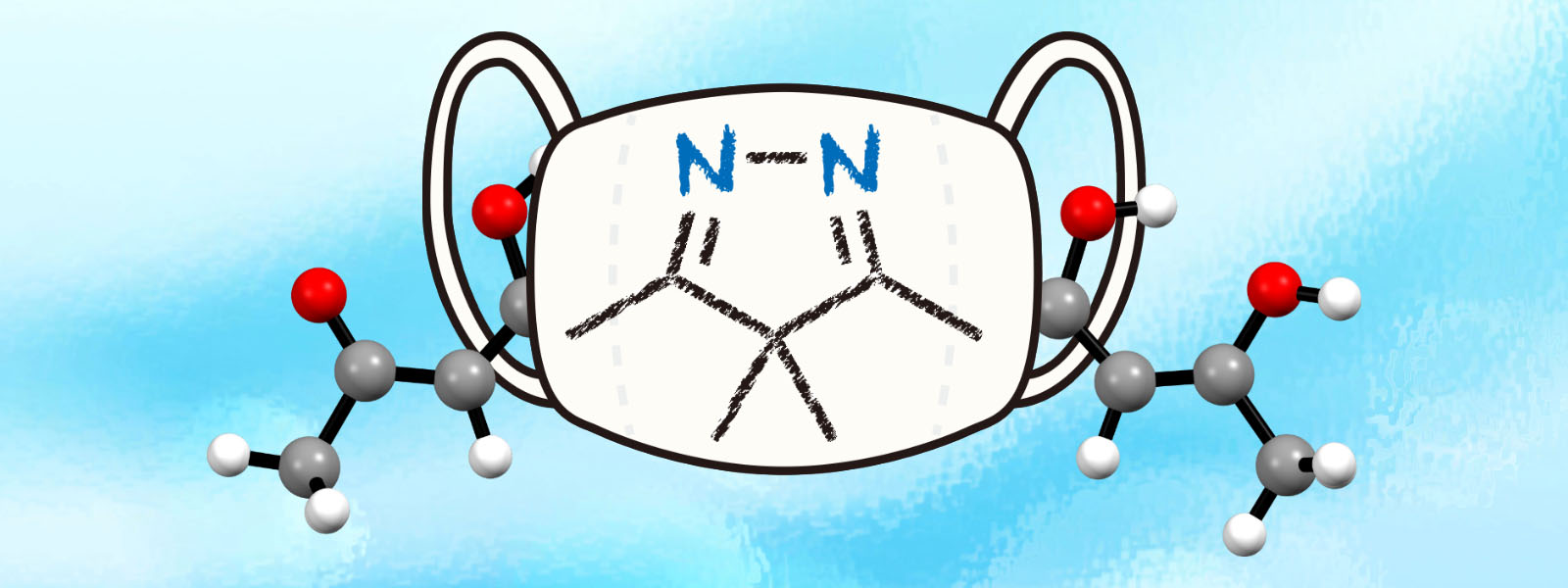 "マスク"をしたカルボニルひもで、発光性分子が出来ました。 |
| 67. | Polyketone-Based Molecular Ropes as Versatile Components for Functional Materials Y. Inokuma, Y. Inaba Bull. Chem. Soc. Jpn. 2021, 94, 2187-2194. 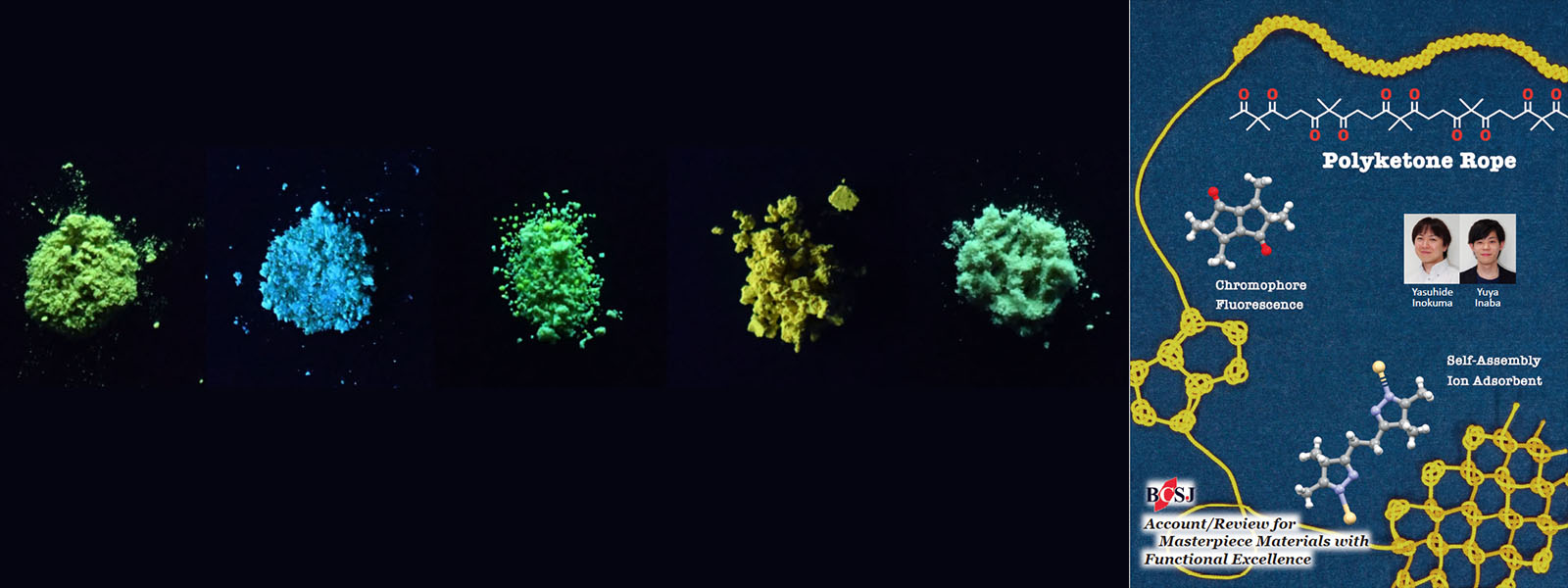 カルボニルひもを使った機能性分子への展開についてAccountを発表しました。 |
| 66. | Insoluble π‐Conjugated Polyimine as an Organic Adsorbent for Group 10 Metal Ions H. Shirakura, Y. Hijikata, J. Pirillo, T. Yoneda, Y. Manabe, M. Murugavel, Y. Ide, Y. Inokuma Eur. J. Inorg. Chem. 2021, 1705-1708. 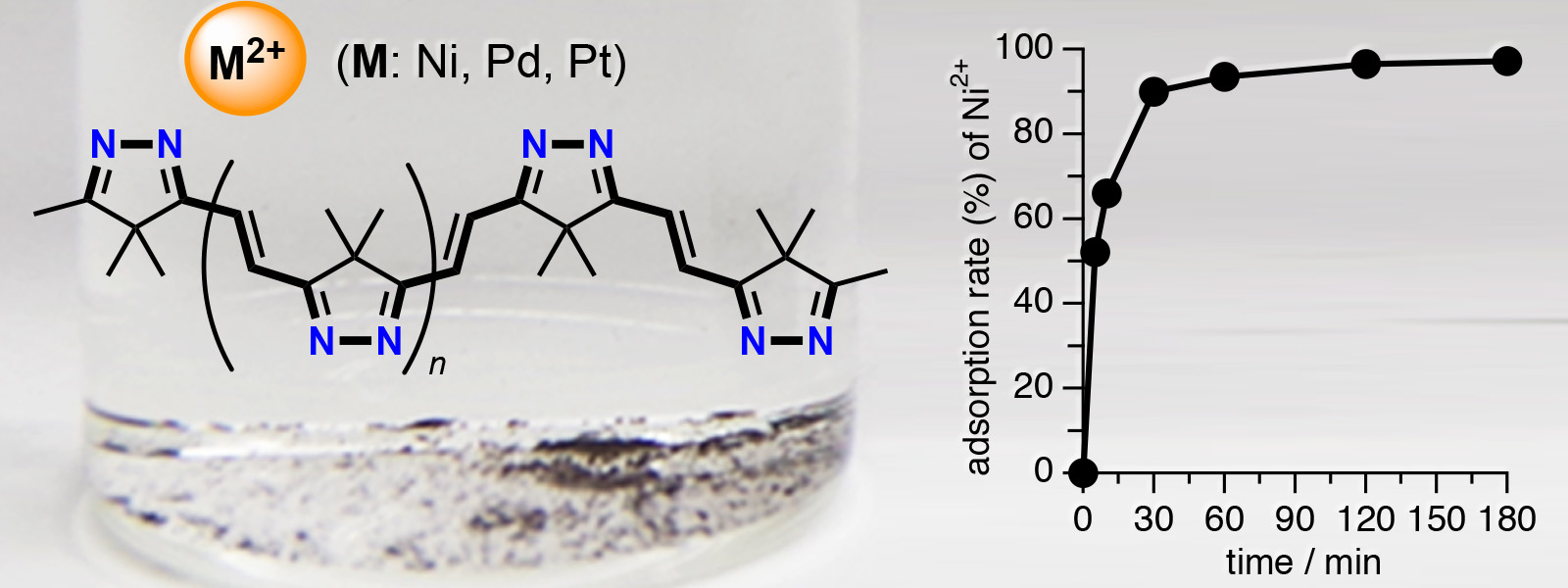 カルボニルひもから誘導される共役ポリイミン化合物に金属吸着の性質が見つかりました。 |
| 65. | Aliphatic Polyketones as Classic yet New Molecular Ropes for Structural Diversity in Organic Synthesis Y. Inokuma, T. Yoneda, Y. Ide, S. Yoshioka Chem. Commun., 2020, 56, 9079-9093. 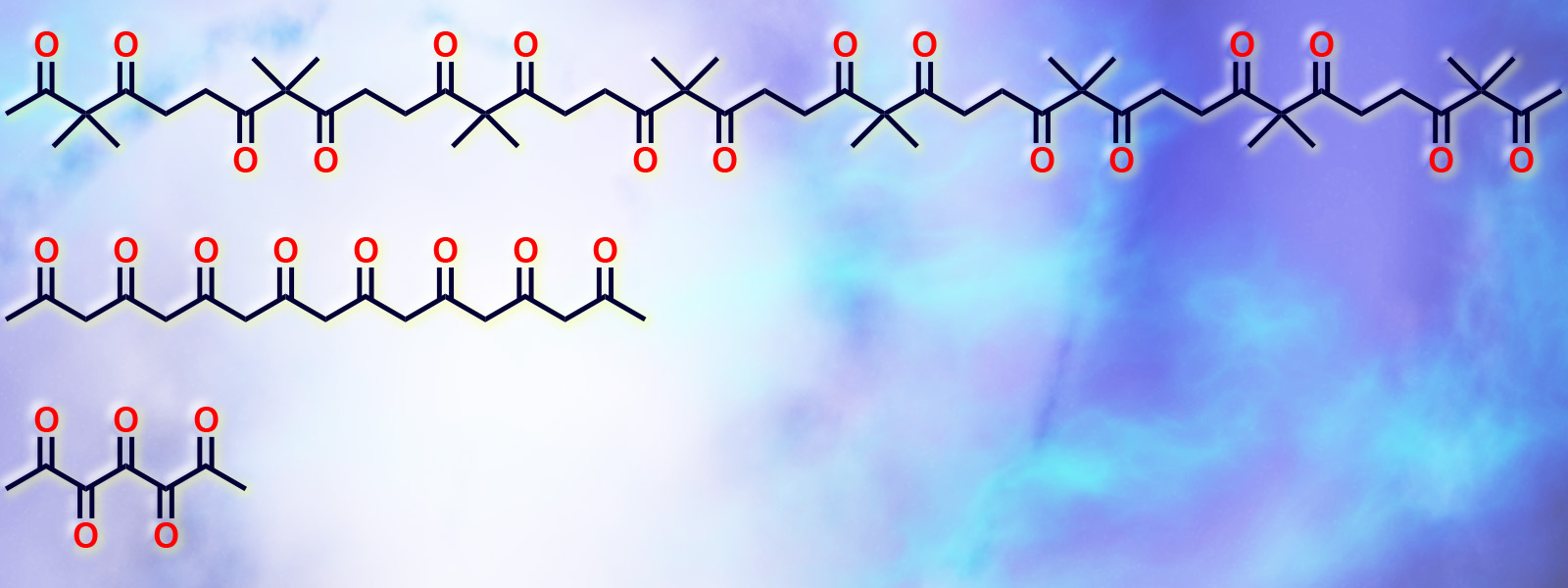 カルボニルひもの概念と脂肪族ポリケトンの合成と反応性に関するFeature Articleを発表しました。 |
| 64. | Spirosilicate Dimers of a Bowl-shaped Diol Generated by Intramolecular Cyclization of an Aliphatic Tetraketone Chains Y. Inaba, Y. Inokuma Chem. Lett. 2020, 49, 882-884. 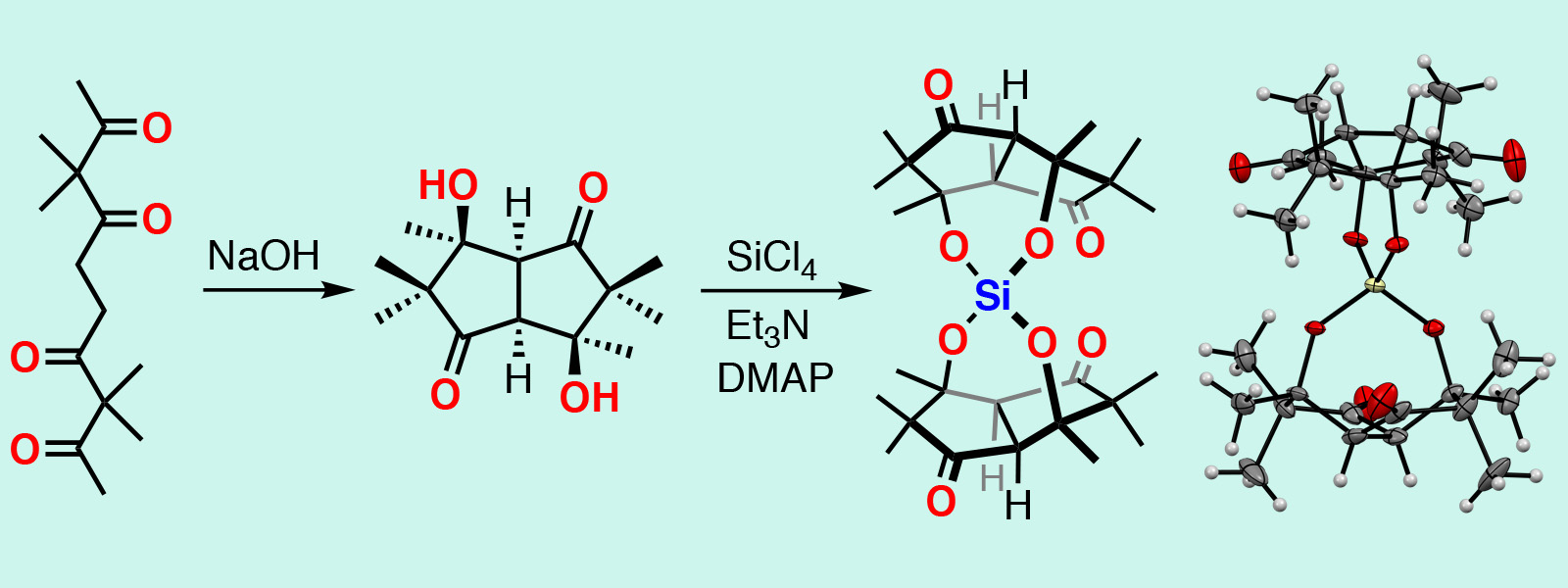 カルボニルひもを使って加水分解に対し安定なスピロシリケートを合成しました。 |
| 63. | Modular Synthesis of Oligoacetylacetones via Site-selective Silylation of Acetylacetone Derivatives P. Sarkar, Y. Inaba, H. Shirakura, T. Yoneda, Y. Inokuma Org. Biomol. Chem. 2020, 18, 3297-3302. 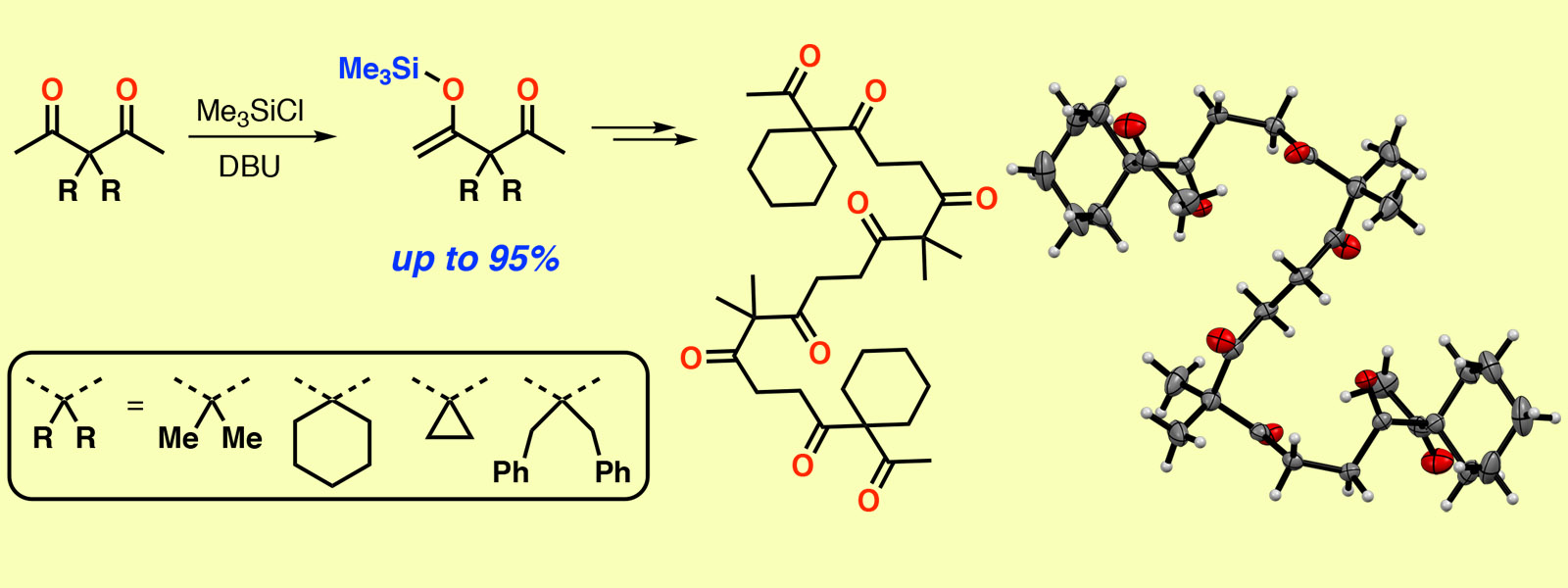 モジュール式合成により様々なシークエンスのカルボニルひもを合成しました。 |
| 62. | Supramolecular Conformational Control of Aliphatic Oligoketones by Rotaxane Formation Y. Manabe, K. Wada, Y. Baba, T. Yoneda, T. Ogoshi, Y. Inokuma Org. Lett. 2020, 22, 3224-3228. 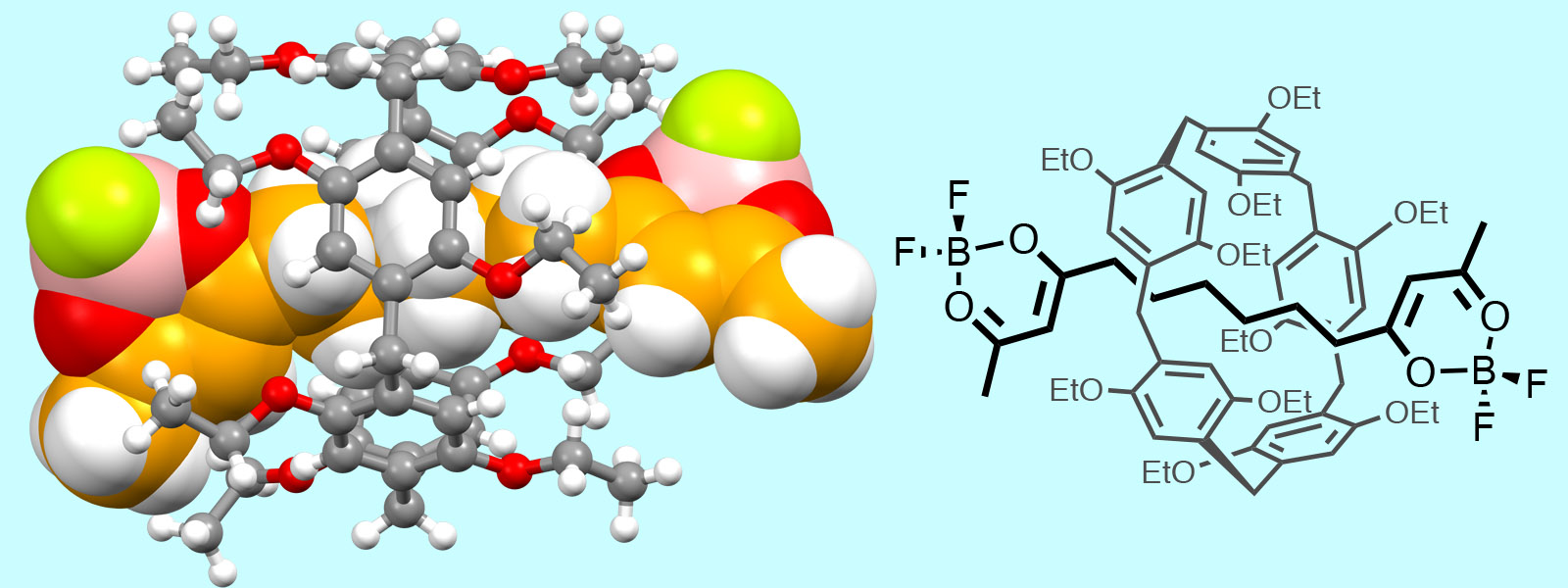 カルボニルひもをピラー[5]アレーンに通してロタキサンを合成しました。京大・生越研究室とのコラボレーションで生まれた成果です。 |
| 61. | Chiral Monolayers with Achiral Tetrapod Molecules on Highly Oriented Pyrolytic Graphite H. Asakawa, S. Matsui, Q. T. Trinh, H. Hirao, Y. Inokuma, T. Ogoshi, S. Tanaka, K. Komatsu, A. Ohta, T. Asakawa, T. Fukuma J. Phys. Chem. C 2020, 124, 7760-7767. 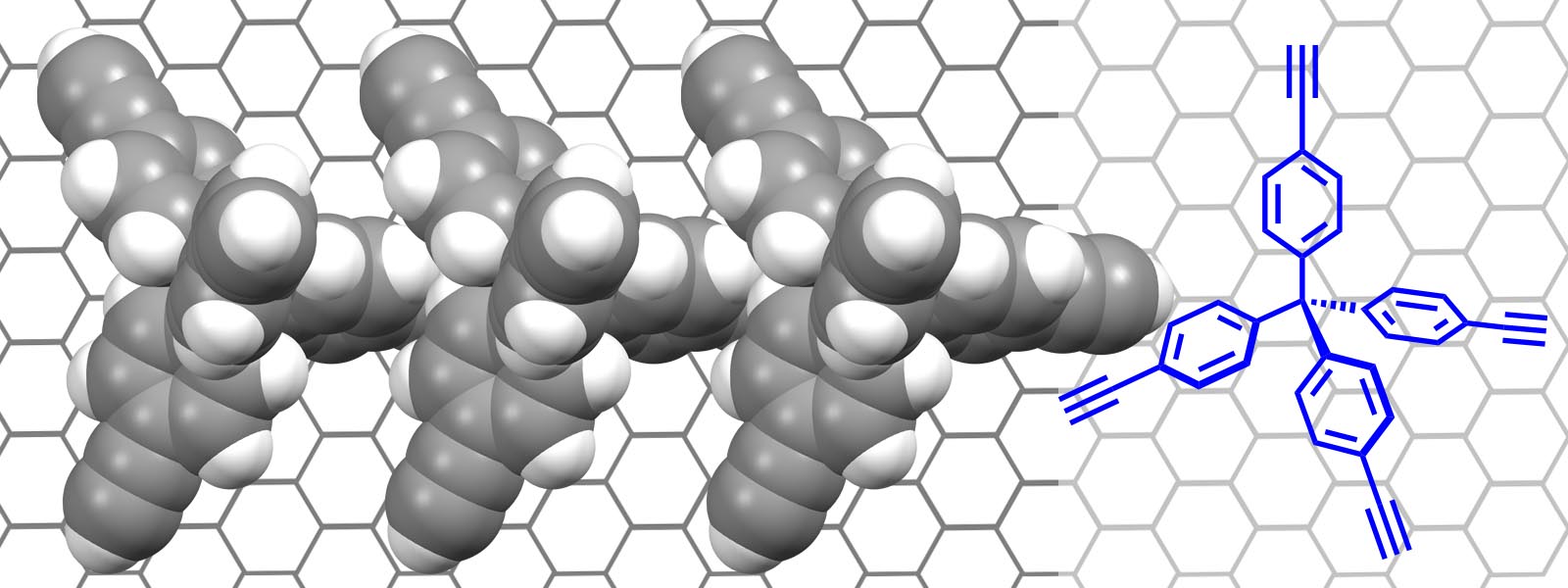 HOPG上にテトラポッド型分子がキラル配列する現象をAFMで観測しました。さきがけ「超空間領域」メンバーの淺川先生、平尾先生、生越先生、そして、金沢大WPI Nano-LSI 福間拠点長をはじめとするメンバーとの共著論文です。 |
| 60. | Polyketones as Host Materials for Solid Polymer Electrolytes T. Eriksson, A. Mace, Y. Manabe, M. Yoshizawa-Fujita, Y. Inokuma, D. Brandell, J. Mindemark J. Electrochem. Soc. 2020, 167, 070537. 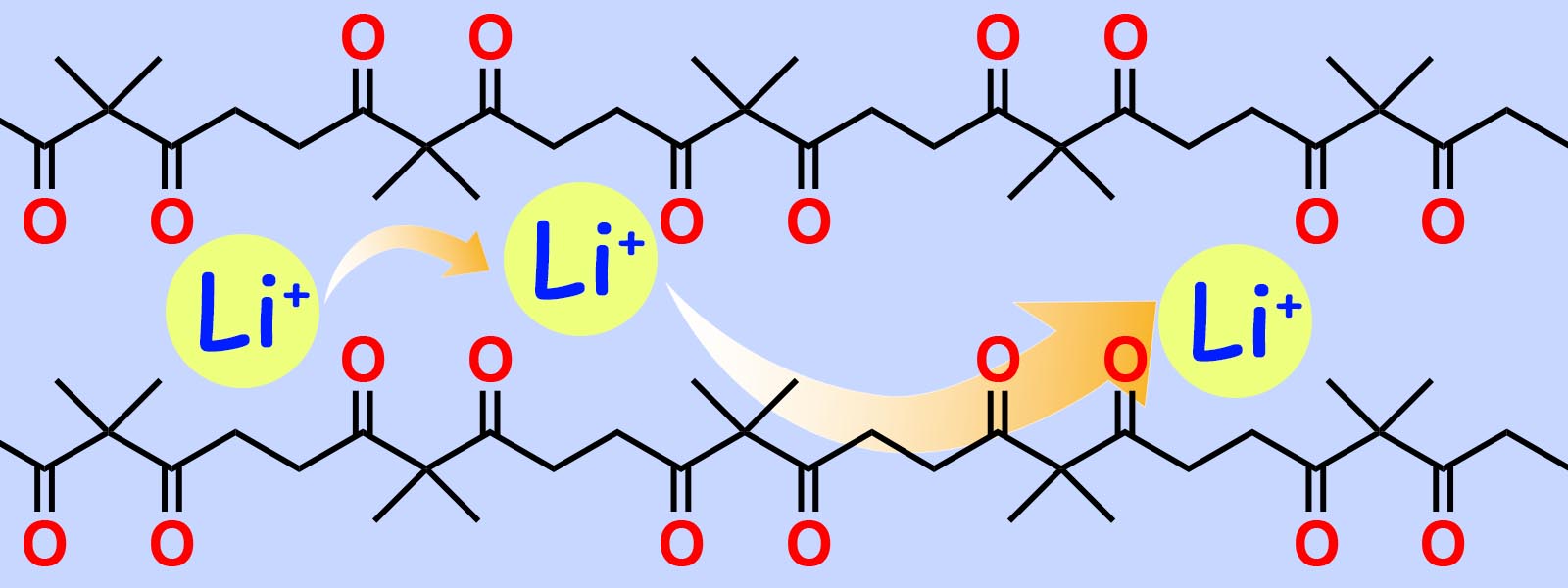 カルボニルひもがリチウムイオン伝導材料として応用可能であることが、スウェーデン・ウプサラ大、上智大との国際共同研究により明らかとなりました。 |
| 59. | Luminescent Coordination Polymers Constructed from Flexible, Tetradentate Diisopyrazole Ligand and Copper(I) Halides T. Yoneda, C. Kasai, Y. Manabe, M. Tsurui, Y. Kitagawa, Y. Hasegawa, P. Sarkar, Y. Inokuma Chem. Asian J. 2020, 15, 601-605. 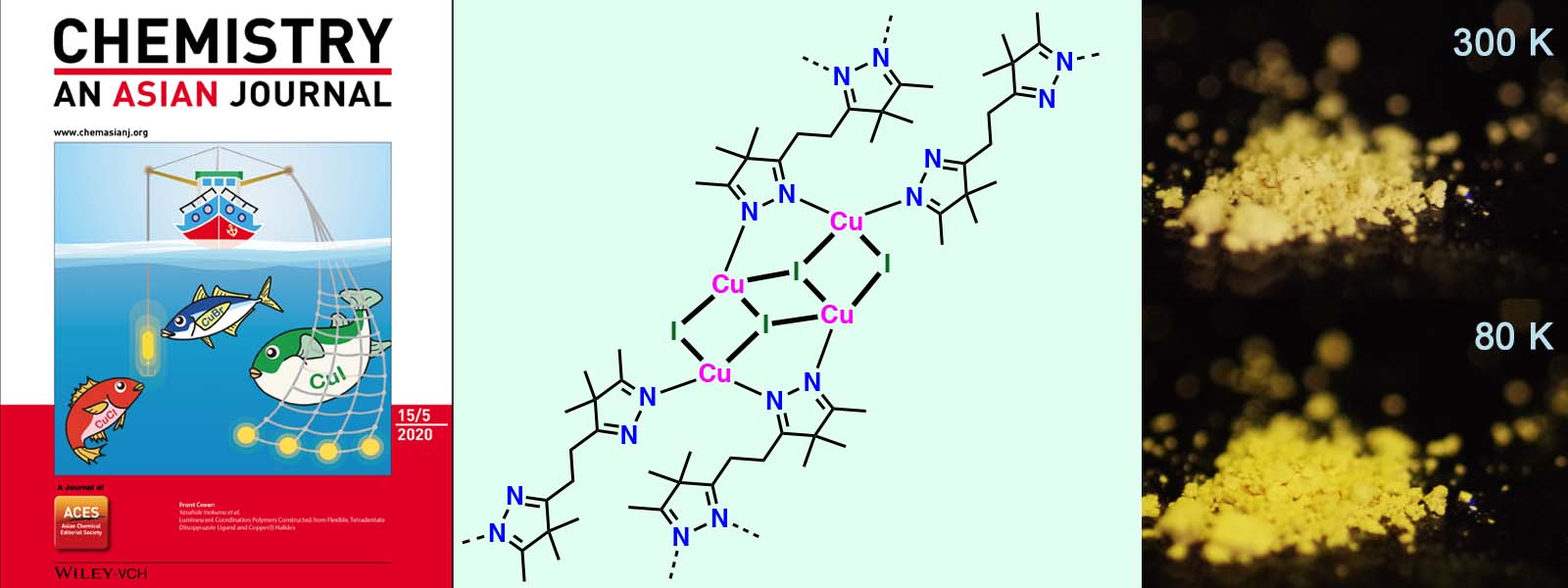 イミンひもとハロゲン化銅(I)から発光性配位高分子を合成しました。特に、ヨウ化銅から得られた錯体は、サーモクロミズムを示しました。ICReDDの北川先生、長谷川先生との共著論文です。Front Coverに採択されました。 |
| 58. | Splitting and Reorientation of π-Conjugation by an Unprecedented Photo-Rearrangement Reaction Y. Inaba, T. Yoneda, Y. Kitagawa, K. Miyata, Y. Hasegawa, Y. Inokuma Chem. Commun. 2020, 56, 348-351. 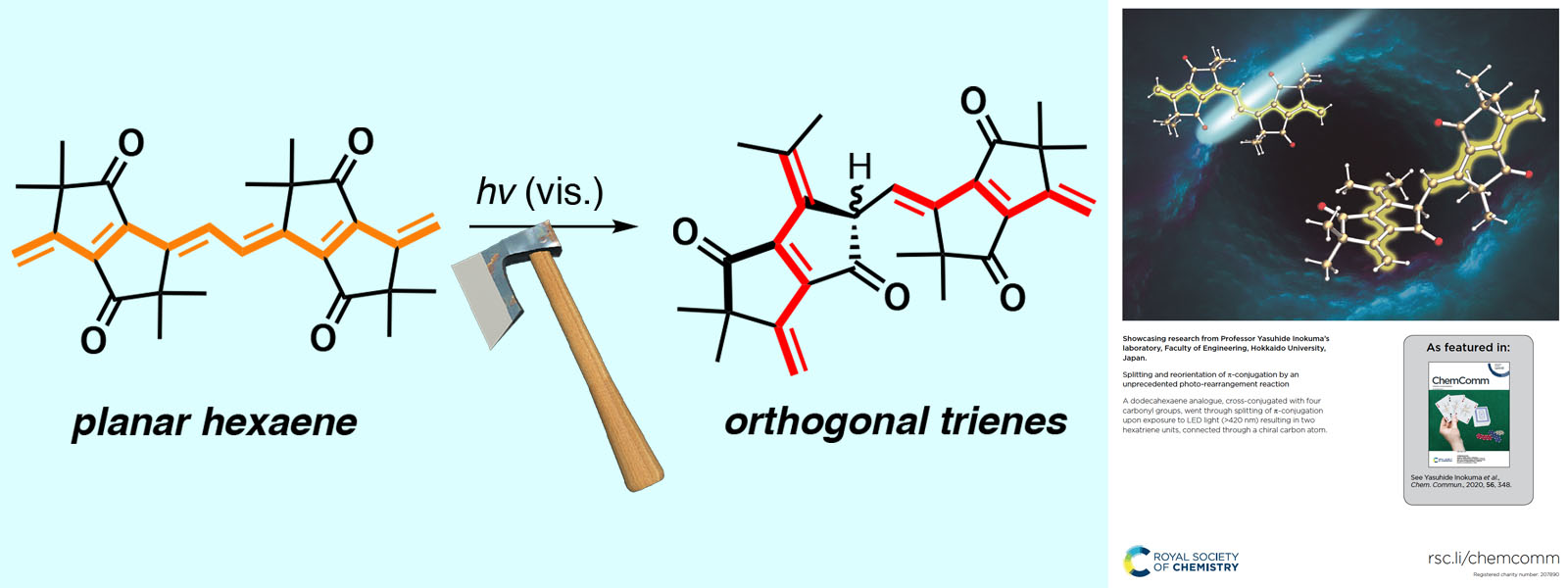 カルボニルひもから誘導したポリエンのπ共役を光で切断し、並べ替える新転位反応を発見しました。ICReDDの北川先生、長谷川先生、そして九大の宮田先生との共同研究論文です。Back Coverに採択されました! |
| 57. | Identification of Actinomycin D as a Specific Inhibitor of the Alternative Pathway of Peptidoglycan Biosynthesis Y. Ogasawara, Y. Shimizu, Y. Sato, T. Yoneda, Y. Inokuma, T. Dairi J. Antibiot. 2020, 73, 125-127. 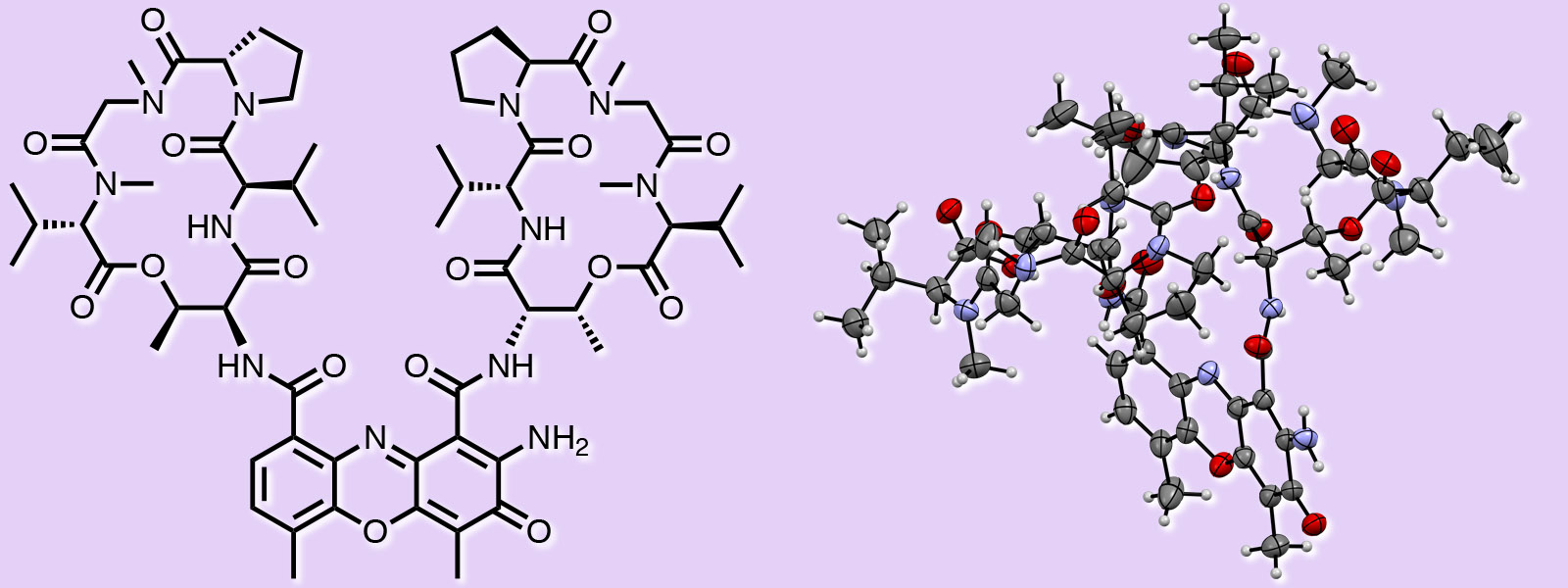 同学科の小笠原先生、大利先生との共著論文です。Actinomycin Dの結晶構造解析をお手伝いさせて頂きました。 |
| 56. | Asymmetric Synthesis of a 5,7-Fused Ring System Enabled by an Intramolecular Buchner Reaction with Chiral Rhodium Catalyst T. Hoshi, E. Ota, Y. Inokuma, J. Yamaguchi Org. Lett. 2019, 21, 10081-10084. 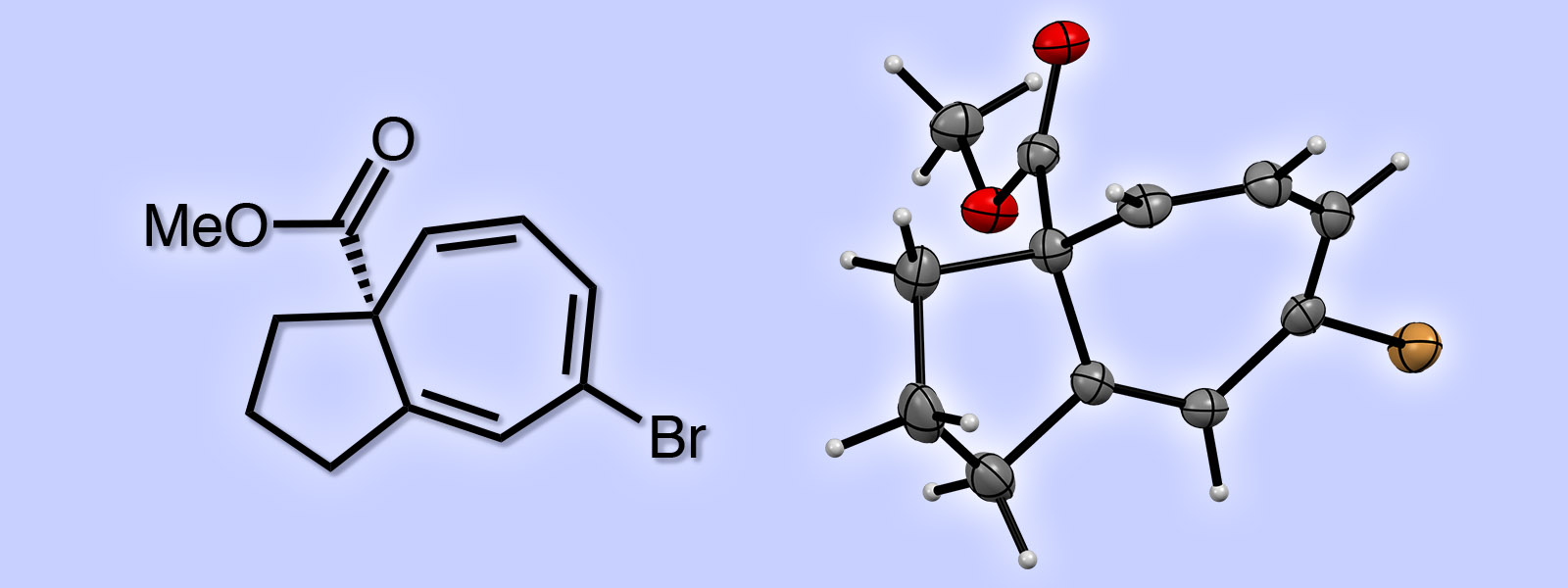 早稲田大学の山口潤一郎先生との共著論文です。不斉合成した生成物の結晶構造解析をお手伝いさせて頂きました。 |
| 55. | Aliphatic Polyketones as Shapable Molecular Chains Y. Inokuma J. Synth. Org. Chem. Jpn. 2019, 77, 1078-1085. 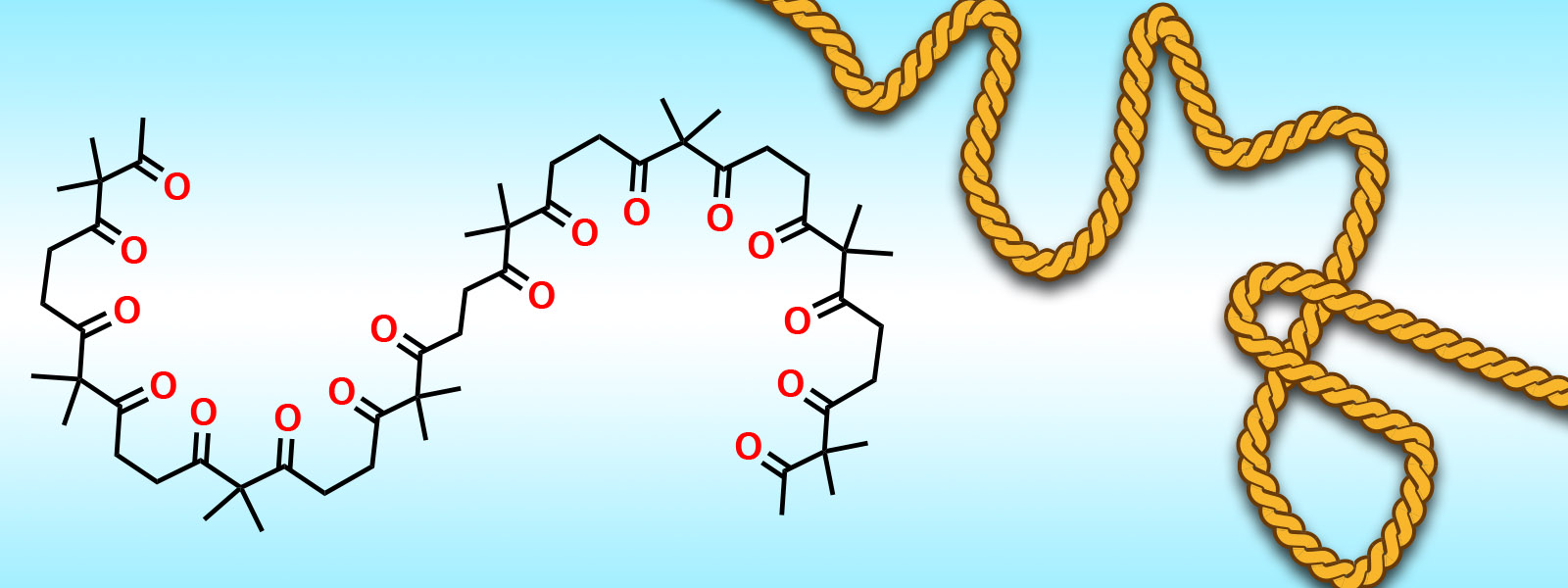 カルボニルひもに関する総合論文です。 |
| 54. | Two-Step Transformation of Aliphatic Polyketones into π-Conjugated Polyimines Y. Manabe, M. Uesaka, T. Yoneda, Y. Inokuma J. Org. Chem. 2019, 84, 9957-9964. 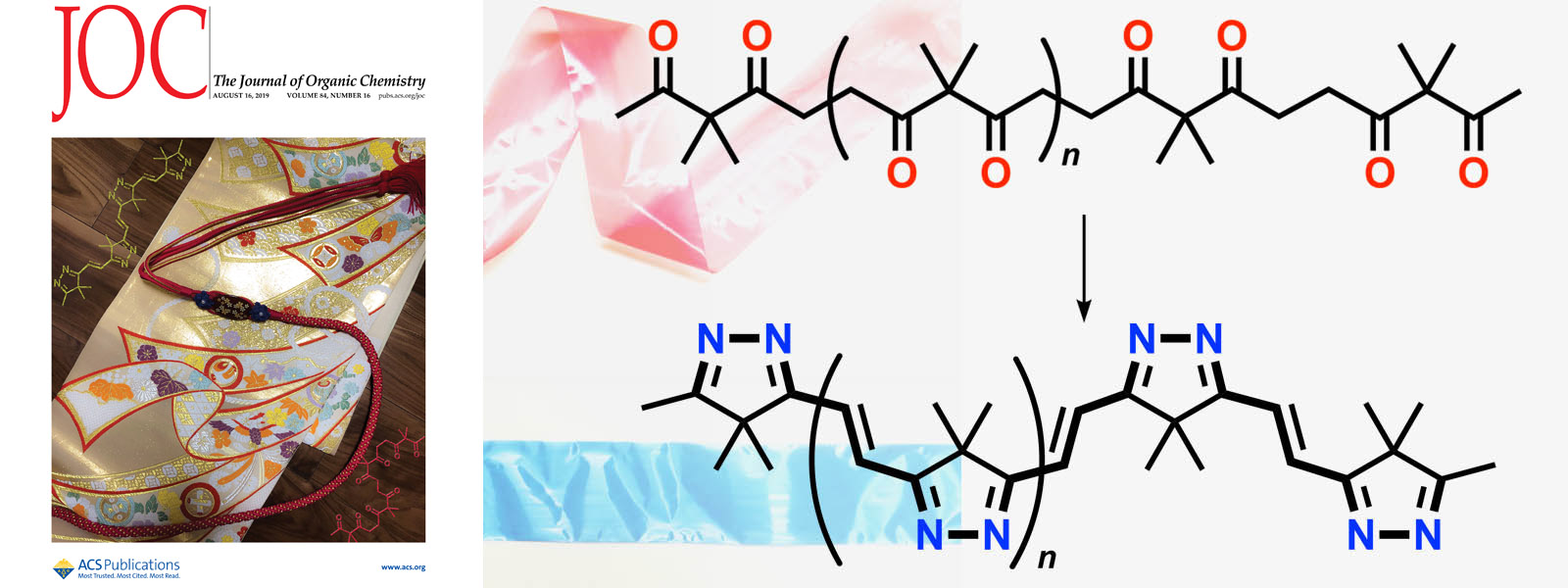 カルボニルひもからπ共役イミン色素の帯ができました。カバーピクチャにも採択されました。 |
| 53. | Control over Coordination Self-Assembly of Flexible, Multidentate Ligands by Stepwise Metal Coordination of Isopyrazole Subunits Y. Ashida, Y. Manabe, S. Yoshioka, T. Yoneda, Y. Inokuma Dalton Trans. 2019, 48, 818-822. 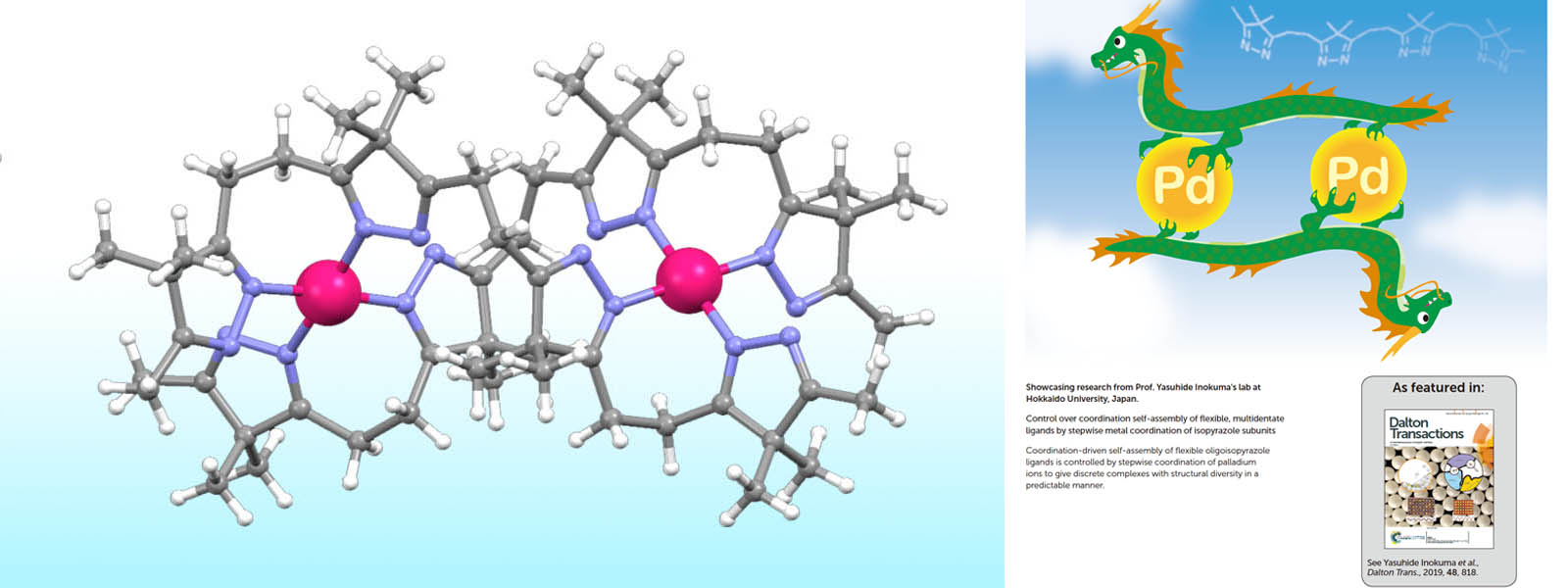 イミンひもをパラジウムイオンとの段階的錯形成を用いて自己集合させることに成功しました。本論文に関連してInside Back Coverに採択されました。 |
| 52. | Bioinspired Synthesis of Pentalene-based Chromophores from an Oligoketone Chain Y. Saito, M. Higuchi, S. Yoshioka, H. Senboku, Y. Inokuma Chem. Commun. 2018, 54, 6788-6791. 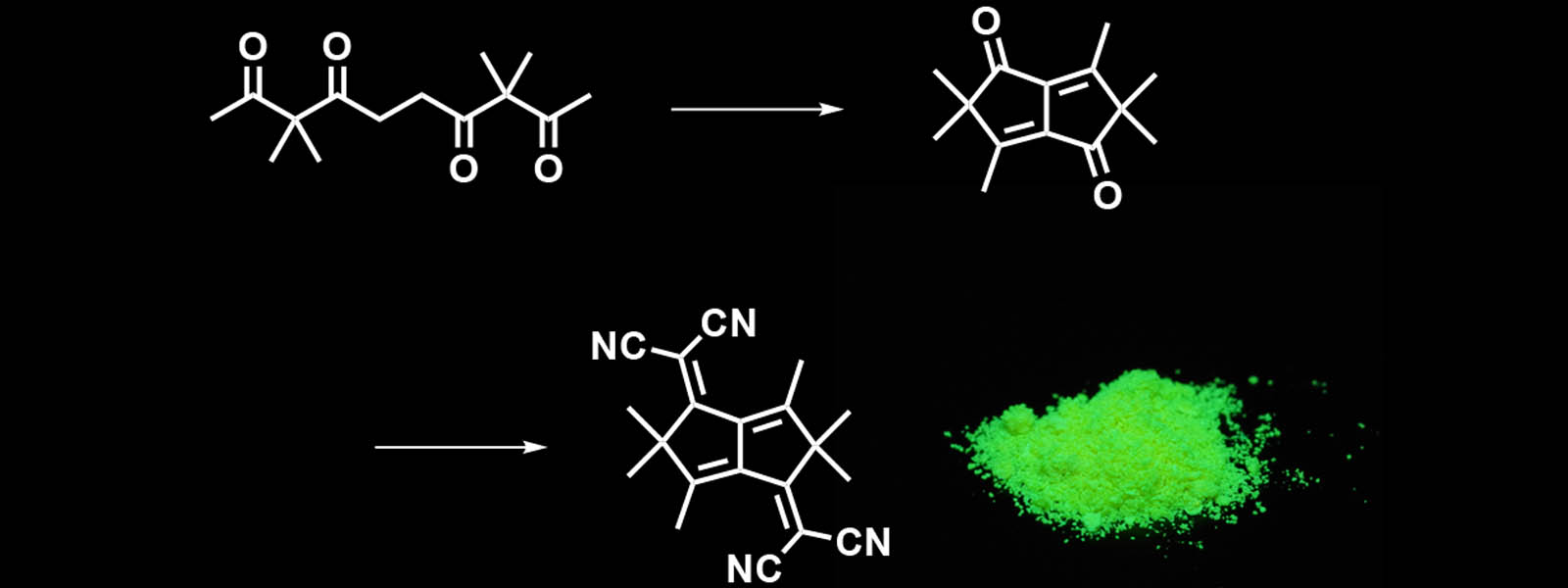 カルボニルひもを折り畳んで固体発光を示す色素に変換した研究です。 |
| 51. | Oligoacetylacetones as Shapable Carbon Chains and Their Transformation to Oligoimines for Construction of Metal-organic Architectures M. Uesaka, Y. Saito, S. Yoshioka, Y. Domoto, M. Fujita, Y. Inokuma Communications Chemistry 2018, 1, Article Number 23.  カルボニルひも化合物のコンセプト、合成、分子集合体への応用を報告した猪熊グループ初の論文です。 北海道大学のプレスリリースは、こちら。 JST版は、こちら。 Behind the Paper (Nature Research Chemistry Community)の紹介記事 |
| 50. | Chiral Crystalline Sponges for the Absolute Structure Determination of Chiral Guests K. Yan, R. Dubey, T. Arai, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2017, 139, 11341-11344. |
| 49. | Structural Elucidation of Trace Amounts of Volatile Compounds Using the Crystalline Sponge Method N. Zigon, T. Kikuchi, J. Ariyoshi, Y. Inokuma, M. Fujita Chem. Asian J. 2017, 12, 1057-1061. |
| 48. | Finding a New Crystalline Sponge from a Crystallographic Database Y. Inokuma, K. Matsumura, S. Yoshioka, M. Fujita Chem. Asian. J. 2017, 12, 208-211. (Front Cover) |
| 47. | X-ray Structure Analysis of Ozonides by the Crystalline Sponge Method S. Yoshioka, Y. Inokuma, V. Duplan, R. Dubey, M. Fujita J. Am. Chem. Soc. 2016, 138, 10140-10142. |
| 46. | A Saccharide-based Crystalline Sponge for Hydrophilic Guests G.-H. Ning, K. Matsumura, Y. Inokuma, M. Fujita Chem. Commun. 2016, 52, 7013-7015. |
| 45. | Structure Determination of Microbial Metabolites by the Crystalline Sponge Method Y. Inokuma, T. Ukegawa, M. Hoshino, M. Fujita Chem. Sci. 2016, 7, 3910-3913. |
| 44. | The Crystalline Sponge Method Updated M. Hoshino, A. Khutia, H. Xing, Y. Inokuma, M. Fujita IUCrJ 2016, 3, 139-151. |
| 43. | Where is the Oxygen? Structural Analysis of a-Humulene Oxidation Products by the Crystalline Sponge Method N. Zigon, M. Hoshino, S. Yoshioka, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2015, 54, 9033-9037. |
| 42. | Absolute Structure Determination of Compounds with Axial and Planar Chirality Using the Crystalline Sponge Method S. Yoshioka, Y. Inokuma, M. Hoshino, T. Sato, M. Fujita Chem. Sci. 2015, 6, 3765-3768. |
| 41. | Networked-Cage Microcrystals for Evaluation of Host-guest Interactions S. Matsuzaki, T. Arai, K. Ikemoto, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2014, 136, 17899-17901. |
| 40. | Visualization of Solution Chemistry by X-ray Crystallography Using Porous Coordination Networks Y. Inokuma, M. Fujita Bull. Chem. Soc. Jpn. 2014, 87, 1161-1176. (Award Article, Back Cover) |
| 39. | Radical C-H Functionalization of Heteroarenes under Electrochemical Control A. O'Brien, A. Maruyama, Y. Inokuma, M. Fujita, P. S. Baran, D. G. Blackmond Angew. Chem. Int. Ed. 2014, 53, 11868-11871. (Selected as a Hot Paper) |
| 38. | X-ray Snapshot Observation of Palladium-Mediated Aromatic Bromination in a Porous Complex K. Ikemoto, Y. Inokuma, K. Rissanen, M. Fujita J. Am. Chem. Soc. 2014, 136, 6892-6895. (Highlighted by "Chemistry World", RSC) |
| 37. | Preparation and Guest-uptake Protocol for a Porous Complex Useful for 'Crystal-free' Crystallography Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, M. Fujita Nature Protocols 2014, 9, 246-252. |
| 36. | Stable Encapsulation of Acrylate Esters in Networked Molecular Capsules G.-H Ning, Y. Inokuma, M. Fujita Chem. Asian J. 2014, 9, 466-468. (VIP paper, Inside Cover) |
| 35. | Unique Ultrafast Energy Transfer in a Series of Phenylene-bridged Subporphyrin-porphyrin Hybrids J. Oh, J. Sung, M. Kitano, Y. Inokuma, A. Osuka, D. Kim Chem. Commun. 2014, 50, 10424-10426. |
| 34. | Dynamic Behavior of M6L4 Capsules in Solution and Crystalline States G.-H Ning, Y. Inokuma, M. Fujita Chem. Asian J. 2013, 8, 2596-2599. |
| 33. | X-ray analysis on the nanogram to microgram scale using porous complexes Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen, M. Fujita Nature 2013, 495, 461-466. (Highlighted by "Nature news and views" ) (Highlighted by "C&EN", ACS) (Highlighted by "Chemistry World", RSC) |
| 32. | Reagent-Installed Capsule Network: Selective Thiocarbamoylation of Aromatic Amines in Crystals with Pre-installed CH3NCS Y. Inokuma, G.-H. Ning, M. Fujita Angew. Chem. Int. Ed. 2012, 51, 2379-2381. |
| 31. | Oxocyclohexadienylidene-Substituted Subporphyrins S. Hayashi, J. Sung, Y. Sung, Y. M. Sung, Y. Inokuma, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2011, 50, 3253-3256. |
| 30. | Bimolecular Reaction via the Successive Introduction of Two Substrates into the Crystals of Networked Molecular Cages Y. Inokuma, N. Kojima, T. Arai, M. Fujita J. Am. Chem. Soc. 2011, 133, 19691-19693. |
| 29. | Diels-Alder via Molecular Recognition in a Crystalline Molecular Flask K. Ikemoto, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2011, 133, 16806-16808. |
| 28. | Shedding Light on Hidden Reaction Pathways in Radical Polymerization by a Porous Coordination Network Y. Inokuma, S. Nishiguchi, K. Ikemoto, M. Fujita Chem. Commun. 2011, 47, 12113-12115. |
| 27. | Synthesis and Properties of Boron(III)-Coordinated Subbacteriochlorins S. Hayashi, E. Tsurumaki, Y. Inokuma, P. Kim, Y. M. Sung, D. Kim, A. Osuka J. Am. Chem. Soc. 2011, 133, 4254-4256. |
| 26. | Crystalline Molecular Flasks Y. Inokuma, M. Kawano, M. Fujita Nature Chem. 2011, 3, 349-358. |
| 25. | A Molecular Capsule Network: Guest Encapsulation and Control of Diels-Alder Reactivity Y. Inokuma, S. Yoshioka, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 8912-8914. |
| 24. | Networked Molecular Cages as Crystalline Sponges for Fullerenes and Other Guests Y. Inokuma, T. Arai, M. Fujita Nature Chem. 2010, 2, 780-783. (Highlighted by "C&EN", ACS) |
| 23. | The Reaction of Organozinc Compounds with an Aldehyde within a Crystalline Molecular Flask K. Ikemoto, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 5750-5752. |
| 22. | The Catalytic Z to E Isomerization of Stilbenes in a Photosensitizing Porous Coordination Network K. Ohara, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 5507-5509. |
| 21. | Regioselective Huisgen Cycloaddition within Porous Coordination Networks T. Kawamichi, Y. Inokuma, M. Fujita Angew. Chem. Int. Ed. 2010, 49, 2375-2377. (Highlighted by "Noteworthy Chemistry", ACS) |
| 20. | A Porous Coordination Network Catalyzes an Olefin Isomerization Reaction in the Pore K. Ohara, M. Kawano, Y. Inokuma, M. Fujita J. Am. Chem. Soc. 2010, 132, 30-31. |
| 19. | meso-Tris(oligo-2,5-thienylene)-Substituted Subporphyrins S. Hayashi, Y. Inokuma, A. Osuka Org. Lett. 2010, 12, 4148-4151. |
| 18. | meso-Trifluoromethyl-substituted Subporphyrin from Ring-splitting Reaction of meso-Trifluoromethyl-substituted [32]Heptaphyrin(1.1.1.1.1.1.1) R. Sakamoto, S. Saito, S. Shimizu, Y. Inokuma, N. Aratani, A. Osuka Chem. Lett. 2010, 39, 439-441. |
| 17. | meso-Trialkyl-substituted Subporphyrins S. Hayashi, Y. Inokuma, S. Easwaramoorthi, K. S. Kim, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2010, 49, 321-324. |
| 16. | Versatile Photophysical Properties of meso-Aryl Substituted Subporphyrins: Dipolar and Octupolar Charge-Transfer Interactions S. Easwaramoorthi, J.-Y. Shin, S. Cho, P. Kim, Y. Inokuma, E. Tsurumaki, A. Osuka, D. Kim Chem. Eur. J. 2009, 15, 12005-12017. |
| 15. | Capped Subporphyrins Y. Inokuma, A. Osuka Chem. Eur. J. 2009, 15, 6863-6876. |
| 14. | 1,4-Phenylene-bridged Subporphyrin-porphyrin Dyad, Triad, and Tetrad Y. Inokuma, S. Hayashi, A. Osuka Chem. Lett. 2009, 38, 206-207. |
| 13. | Peripheral Hexabromination, Hexaphenylation, and Hexaethynylation of meso-Aryl- Substituted Subporphyrins E. Tsurumaki, Y. Inokuma, S. Easwaramoorthi, J. M. Lim, D. Kim, A. Osuka Chem. Eur. J. 2009, 15, 237-247. |
| 12. | 3,3- and 4,4-Biphenylene-Bridged Subporphyrin Dimers Y. Inokuma, A. Osuka Org. Lett. 2008, 10, 5561-5564. |
| 11. | Unambiguous Identification of Möbius Aromaticity for meso-Aryl-Substituted [28]Hexaphyrins(1.1.1.1.1.1) J. Sankar, S. Mori, S. Saito, H. Rath, M. Suzuki, Y. Inokuma, H. Shinokubo, K. S. Kim, Z. S. Yoon, J.-Y. Shin, J. M. Lim, Y. Matsuzaki, O. Matsushita, A. Muranaka, N. Kobayashi, D. Kim, A. Osuka J. Am. Chem. Soc. 2008, 130, 13568-13579. |
| 10. | meso-(4-(N,N-Dialkylamino)phenyl)-Substituted Subporphyrins: Remarkably Perturbed Absorption Spectra and Enhanced Fluorescence by Intramolecular Charge Transfer Interactions Y. Inokuma, S. Easwaramoorthi, Z. S. Yoon, D. Kim, A. Osuka J. Am. Chem. Soc. 2008, 130, 12234-12235. |
| 9. | Effective Expansion of the Subporphyrin Chromophore Through Conjugation with meso-Oligo(1,4-phenyleneethynylene) Substituents: Octupolar Effect on Two-Photon Absorption Y. Inokuma, S. Easwaramoorthi, S. Y. Jang, K. S. Kim, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2008, 47, 4840-4843. |
| 8. | Subporphyrins: Emerging Contracted Porphyrins with Aromatic 14π-Electronic Systems and Bowl-Shaped Structures: Rational and Unexpected Synthetic Routes Y. Inokuma, A. Osuka Dalton Trans. 2008, 2517-2526. (Perspective, Front Cover) |
| 7. | Synthesis and Characterization of meso-Aryl-Substituted Subchlorins E. Tsurumaki, S. Saito, K. S. Kim, J. M. Lim, Y. Inokuma, D. Kim, A. Osuka J. Am. Chem. Soc. 2008, 130, 438-439. |
| 6. | Complementary Face-to-Face Dimer Formation From meso-Aryl Subporphyrins Bearing a 2-Carboxyphenyl Group Y. Inokuma, A. Osuka Chem. Commun. 2007, 2938-2940. |
| 5. | meso-Aryl-Substituted Subporphyrins: Synthesis, Structures, and Large Substituent Effects on Their Electronic Properties Y. Inokuma, Z. S. Yoon, D. Kim, A. Osuka J. Am. Chem. Soc. 2007, 129, 4747-4761. |
| 4. | Tribenzosubporphines: Synthesis and Characterization Y. Inokuma, J. H. Kwon, T. K. Ahn, M.-C. Yoon, D. Kim, A. Osuka Angew. Chem. Int. Ed. 2006, 45, 961-964. |
| 3. | Enlarged π-Electronic Network of a meso-meso, β-β, β-β Triply Linked Dibenzoporphyrin Dimer that Exhibits a Large Two-Photon Absorption Cross Section Y. Inokuma, N. Ono, H. Uno, D. Y. Kim, S. B. Noh, D. Kim, A. Osuka Chem. Commun. 2005, 3782-3784. |
| 2. | A Doubly N-Fused Benzohexaphyrin and Its Rearrangement to a Fluorescent Macrocycle upon DDQ Oxidation Y. Inokuma, T. Matsunari, N. Ono, H. Uno, A. Osuka Angew. Chem. Int. Ed. 2005, 44, 1856-1860. |
| 1. | meso-Porphyrinyl-Substituted Porphyrin and Expanded Porphyrins Y. Inokuma, A. Osuka Org. Lett. 2004, 6, 3663-3666. |
