- HOME
- Wow! Cool Laboratory [Researcher Introduction]
- Akira Miura
Wow! Cool Laboratory [Researcher Introduction]

Division of Applied Chemistry,
Research Group of Inorganic Materials Chemistry,
Inorganic Synthesis Chemistry Laboratory
Field of research: inorganic chemistry, materials science
Research themes: synthesis reactions, nitride synthesis
E-mail: amiura[a]eng.hokudai.ac.jp
Demonstrating the process behind the synthesis reaction of yttrium barium copper oxide (YBCO), a high-temperature superconductor, through international collaborative research by seven universities and one business institution from the U.S. and Japan
In-situ measurement and introduction of a computational model for establishing a more reliable research system
The Inorganic Synthesis Chemistry Laboratory has been working to create high-performance ceramics that can solve environmental and energy problems. Dr. Akira Miura, an associate professor who received the Hokkaido University Graduate School and Faculty of Engineering Young Faculty Award in March 2021, has been trying to elucidate the ceramic synthesis process in a furnace, which is regarded as a black box, by utilizing in-situ measurements and computational modeling.
Ceramics are used in solid-oxide fuel cells, semiconductors, and various functional and energy materials. Many of these ceramics are compositionally complex and their synthesis processes have yet to be understood.
“In ceramic synthesis, several types of raw powders are heated to temperatures higher than hundreds of degrees in order to promote a reaction, but there are limited means for visually confirming the reaction process in the electric furnace. Computational science helps compensate for the lack of those means. The current worldwide trend is toward more reliable research methods that don’t overburden researchers, rather than traditional research methods that require researchers to make lots of trial-and-error efforts. By using a computation model, the number of possible combinations, which would otherwise be infinite, can be reduced. In-situ measurements using electron microscopy and radiant light to elucidate the reaction mechanism make it possible to develop theoretical reaction designs. Consequently, the period needed to create new functional materials can be significantly shortened.”
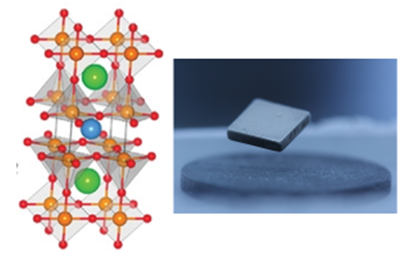
Fig. 1 Crystalline structure of YBa2Cu3O7-x, a high-temperature superconductor, and a photo showing electromagnetic suspension
An international collaborative research network in which expertise is shared
Direct Observation and Computational Modeling of Solid-State Ceramics Synthesis, a research project of Associate Professor Miura that was announced in a press release in May 2021, is the result of international collaborative research involving seven universities and one business institution from the U.S. and Japan. The ceramic material used in the research is YBCO (yttrium barium copper oxide), a high-temperature superconductor synthesized by heating a mixture of powdered yttrium oxide (Y2O3), barium peroxide (BaO2), and copper oxide (CuO) to between 800 and 1,000 degrees (Fig. 2).
Leading to the collaborative research was a discussion at the Gordon Research Conference in the U.S. with Dr. Wenhao Sun (assistant professor of Materials Science & Engineering at the University of Michigan from 2020) regarding synthesis reaction design. Dr. Chris Bartel and Professor Gerbrand Ceder in the Department of Materials Science & Engineering, University of California, Berkeley, contributed to the computational work. Dr. Masanori Nagao, an associate professor at the Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, contributed to the experiment planning stage.
Experiments at SPring-8, a large synchrotron radiation facility, were conducted with the cooperation of Dr. Yosuke Goto, an assistant professor at the Faculty of Science, Tokyo Metropolitan University, and Dr. Yoshihiro Kuroiwa, a professor in the Graduate School of Advanced Science and Engineering, Hiroshima University. Dr. Manabu Shirai from Hitachi High-Tech Corporation has been committed to making electron microscopy observations in order to elucidate what happens in real space.

Fig. 2
The international collaborative research has elucidated the synthesis mechanism. In the early phase of the YBCO synthesis reaction, BaO2, which has the largest reaction energy of the three ceramic materials, reacts with CuO to generate an intermediate product, and in the later phase of the reaction, the intermediate melts and reacts with Y2O3, resulting in YBCO synthesis.
Selected for the PRESTO program, an opportunity to forge a new feature
“The key to successful international collaborative research is networking,” says Dr. Miura. “It’s also necessary to have open-mindedness and a positive attitude toward things that seem interesting, without dismissing new things.”
Based on the results of collaborative research, a new project called New Metastable Materials Designed via Transport Difference has already started. This research was selected for the FY 2021 PRESTO (Precursory Research for Embryonic Science and Technology) program of the Japan Science and Technology Agency (JST) (Fig. 3).
“The intermediate product obtained in the YBCO synthesis reaction provided me with a clue to a new research avenue that focuses on new metastable materials generated in the synthesis process. These materials could have yet-unknown features.” Miura will continue to address new challenges in cooperation with his co-researchers from within and beyond Hokkaido University.
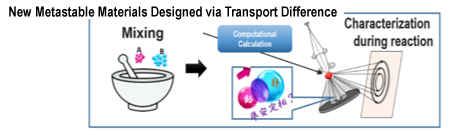
Fig. 3
By combining computational science with in-situ analysis, Miura continues to aim at the creation of new metastable materials, while at the same time trying to elucidate synthesis mechanisms of functional materials such as superconductors and ion conductors.
“Even during the pandemic, collaborative research has been possible online, although these days I actually appreciate the benefits of face-to-face communication, because direct exchanges of information provide me with inspiration.”
