- HOME
- Wow! Cool Laboratory [Researcher Introduction]
- Tsutomu Uchida
Wow! Cool Laboratory [Researcher Introduction]

Division of Applied Physics,
Research Group of Complex Material Physics,
Laboratory of Nanobiotechnology
Field of research: physics, biophysics/chemical physics, nano/micro science, nanomaterials engineering, basic chemistry, physical chemistry
Research theme: physical properties of water, ice and hydrate crystals
E-mail: t-uchida[a]eng.hokudai.ac.jp
Research into water, which is familiar but also mysterious
Striving to reveal the mysteries of water in different disciplines, including physics, biology and chemistry
Exploration of the diverse phenomena of various solid phases of water
Associate Professor Tsutomu Uchida at the Laboratory of Nanobiotechnology, Research Group of Complex Material Physics has been studying ice and clathrate hydrate crystals, triggered by research on Antarctic ice. Clathrate hydrates are crystalline compounds of water and guest molecules, such as methane hydrate (note 1) existing in the deep ocean floor. Associate Professor Uchida is engaged in research on water, ice and clathrate hydrate crystals in different disciplines ranging from geoscience to applied physics, chemistry and biology.
“Water is present universally on earth and indispensable for living organisms to survive. However, water has a highly specific nature, and is a difficult substance for researchers. I have been studying water and ice since I fell under the spell of air hydrate (fossil air) in Antarctic ice.”
He is now engaged in researches on cell cryopreservation technology and micro/nano bubbles. There are increasing expectations for cell cryopreservation technology, which is used for the frozen storage of sperm, eggs and organs. However, the cryopreservation of cells, such as neurons and cardiomyocytes, is still difficult. Associate Professor Uchida is developing technology that makes it possible to cryopreserve these cells by controlling the freezing of water in them.
“Generally, cryopreservation studies are conducted by slowing the speed of freezing, by making crystals smaller or by vitrifying the intra- and inter-cellular water. However, no one knows the actual state of water in the cell yet. So, I am now working on the elucidation of the roles of water in the cryopreservation process.”
To control the freezing process of water in cells, he aims to develop technology to use sucrose, trehalose and other natural anti-freezing substances used by living organisms as natural cryoprotective agents instead of glycerin and other chemicals that have commonly been used.
“Water is a substance indispensable for life. Living organisms skillfully use the nature of water. So, we think that we can control biological activities by controlling the properties of water.”
The world’s first capture of an image of micro/nano bubbles absorbing impurities
Research on micro/nano bubbles is his another themes, which was derived from the study on the freezing of a trehalose solution mentioned above.
“We wanted to observe the freezing process of water precisely at the molecular level, but electron microscopes are not very effective for analyzing water. We thus used the freeze-fracture replication method (a sampling method using the quenching process) to observe the relationship between trehalose and ice. A researcher specializing in fine bubbles who read our paper approached us hoping to use the method to observe fine bubbles, so we got involved in a collaboration.”
This research involves the observation of micro/nano bubbles, which are used for wastewater purification in chemical plants and other facilities. The research team used the freeze-fracture replication method to capture an image of nanobubbles absorbing impurities onto their surface for the first time in the world (note 2).

Artificially generated micro/nano bubbles. The white part in the upper half shows floating microbubbles. Smaller sized fine bubbles are also present in the lower transparent part.
“The life time of nanosize bubbles is said to be less than one second theoretically. However, since it is known that nanobubbles have several special properties, such as the purification effect, how long and how many bubbles exist and how they move are yet to be clarified. Since some bubbles would be formed during freezing, we should develop the quantitative technology with the freeze-fracture replication method to reveal the mechanism of nanobubbles absorbing impurities.”
Technology to use fine bubbles is also attracting attention overseas. In some fields, the application of the technology is underway without clarifying its mechanism and process. He intends to undertake research on the basic mechanism, including the life time of the nanobubbles and its rate-determining process and why they can continue to exist.
“Water exists anywhere, but is a very difficult material to control because it is used to dissolve various substances with complex movements. If we use other liquids to study bubbles, for example, we will be able to use various measurement methods to learn more about bubbles. I think, however, that we may see a new perspective of fine bubbles through water. We will be able to discover new important knowledge on not only the properties of fine bubbles but also the cryopreservation of living cells, if we can clarify the physical properties of water and how water is involved in various phenomena.”
| Note 1. | Methane hydrate Methane hydrate is a crystalline clathrate compound in which methane molecules are surrounded by water molecules under a high pressure and low temperature condition. Methane molecules are trapped in a cage structure, and methane gas is released when the crystal melts. If methane hydrate is put near fire, the released gas burns, so it seems to be “burning ice.” Methane hydrate is considered to exist in great abundance under the deep seabed, and is expected to be utilized as a new natural-gas source. 
|
|---|---|
| Note 2. | Transmission electron microscopic image of micro/nano bubbles in waste water Impurities are absorbed on the surface of nano-bubbles, and the surrounding water is purified. Source: T. Uchida, S. Oshita, M. Ohmori, T. Tsuno, K. Soejima, S. Shinozaki, Y. Take, K. Mitsuta, Nanoscale Research Letters, 6 (1), 295, 2011. (DOI: 10.1186/1556-276X-6-295) 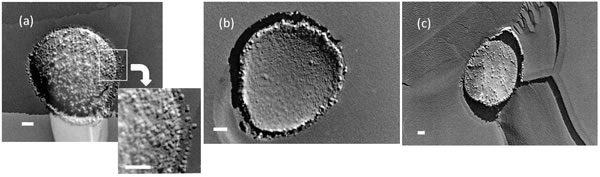
|
