- HOME
- Wow! Cool Laboratory [Researcher Introduction]
- Hiroki Habazaki
Wow! Cool Laboratory [Researcher Introduction]

Division of Applied Chemistry,
Research Group of Functional Materials Chemistry,
Laboratory of Interfacial Electrochemistry
Field of research: functional materials chemistry, electrochemistry, materials surface chemistry
Research theme: preparation of functional oxide films through anodic oxidation, template synthesis of functional nanomaterials
E-mail: habazaki[a]eng.hokudai.ac.jp
Preparation of a nanostructure on the surface of metals through electrochemistry
Applicable to various daily products
Water and oil roll and jump.
Development of a super hydrophobic and oleophobic surface
The boundary between two phases such as liquids and solids is referred to as an interface. Electrochemisty can be utilized to form various functional materials at the interface between a metal and a liquid. Professor Hiroki Habazaki of the Laboratory of Interfacial Electrochemistry is engaged in research and development on functional materials to solve environmental, energy and resource issues. He uses electrochemical and chemical techniques to synthesize functional oxide nanofilms, nanoporous oxide films and nanomaterials.
“The merit of electrochemistry is that you can easily prepare substances at low cost with just an electric source, solution and electrodes. Particularly, it allows you to create thin films in which nano-size pores are regularly arranged much more easily than using a lithographic technique. An abundance of materials such as aluminum and titanium are readily available, and it is easy to design the shape and size of the nanoporous oxides by controlling anodizing conditions. Using this technology, our laboratory is engaged in the development of materials, which are of potential importance for practical applications, including super hydrophobic materials and fuel cells.”
One of the achievements has been the fabrication of super hydrophobic and oleophobic aluminum surface. Professor Habazaki was inspired by the way the surface of lotus leaves repels water to develop a micro/nanoporous thin film that has the same hierarchical structure as lotus leaves (note 1).
“This was accidentally discovered by a student during an experiment, and we found that a hierarchical structure created on the niobium surface has super hydrophobicity that even repels water droplets. We improved it to create that structure on aluminum, and successfully created an aluminum surface, which is more practically important metal than niobium, with the same function.”
This hierarchical structure not only repels water but also oil, and hence, has an anti-fouling/self-cleaning property. The structure can be applied to aluminum foil and transferred to plastic, making it possible to apply anti-fouling surface. Such surface will be useful in daily life.
“Because we use only simple and cost-effective processes for fabrication of super hydrophobic and super oleophobic surface, this technology can be utilized readily in various industries. Snow-repellent structural objects can be created in snowy regions. I expect that there will be innumerable application areas thanks to this water/oil/dirt-repellent property alone.”
It is also possible to create hydrophilic and oleophilic properties, the opposite of super hydrophobic and oleophobic properties, which will lead to a wide range of possible applications. In addition to aluminum, the laboratory has been working on its application to titanium and other light metals as well as iron and stainless steel, and is already advancing collaborative research with a number of companies.
Laminate-structured carbon nanofiber to improve the performance of lithium-ion batteries
Another noteworthy achievement is the development of a carbon material used for lithium ion secondary batteries. The anodic oxidation of an aluminum sheet in an acid solution naturally creates an aluminum oxide film with innumerable 10 to 100-nm cylindrical holes (anodic oxide alumina film). If this film is used as a template, a carbon nanofiber with a special property can be synthesized.
“The major difference from carbon nanotubes is that carbon nanotubes are cylindrical but our carbon nanofibers have hexagonal carbon layers aligned in the axial direction of the fibers. In other words, they have a structure like layered floor cushions. The edge state is very important for carbon materials, and the charge-discharge efficiency varies depending on edge treatment. The carbon nanofiber developed this time has a better edge state than traditional ones, enabling high-rate charging and discharging. It makes it possible to improve the characteristics of lithium-ion batteries tremendously (note 2).”
Carbon materials are also used for fuel cells. In existing fuel cells, platinum as a catalyst is attached to carbon that is a platinum support. Since platinum is an expensive metal, the key to cost management is knowing how to improve its dispersion on carbon.
“We have found that the carbon nanofiber we developed not only has the property of readily supporting platinum, but also is excellent in durability and highly effective as electrodes of fuel cells.”
Only a small number of carbon nanofibers can be manufactured with manufacturing methods that use an anodic oxidation alumina film template. Accordingly, the practical application of carbon nanofibers using the current method is difficult. However, it is a great achievement that Professor Habazaki has clarified the superiority of carbon nanofibers that have a laminate structure with lots of edges. The professor has said that he would search for industry-academia collaboration toward the development of a manufacturing method that enables the mass production of carbon nanofibers.
“It is not easy at all to synthesize carbon with such accurate lamination layers. It is also hard to predict the orientation of the carbon we are developing. However, we are making all kinds of efforts through trial and error based on the data we have accumulated. It has gradually become clear that interfacial interaction is very important to control the orientation. Diverse functions may be possible by using different metals and oxides. Our research still remains at the basic level, but we have found that this technology has outstanding properties. We will pursue the way to put it in practical application.”
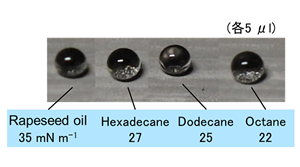
Photo 1. Super hydrophobic aluminum surface

Photo 2. Laboratory
