- HOME
- Wow! Cool Laboratory [Researcher Introduction]
- Yasuchika Hasegawa
Wow! Cool Laboratory [Researcher Introduction]

Division of Applied Chemistry,
Research Group of Functional Materials Chemistry,
Laboratory of Advanced Materials Chemistry
Field of research: photochemistry, coordination chemistry, nano-inorganic chemistry, organic functional materials
Research theme: the research and development of luminescent metal complexes based on photo-physical chemistry
E-mail: hasegaway[a]eng.hokudai.ac.jp
Hasegawa’ Three Theories defy common wisdom.
Rare earth complexes open the way to the development of revolutionary materials.
The hybrid structure of rare earth ions and organic matter increases the intensity of luminescence a hundred fold.
Applied chemistry is a research discipline where the power of chemistry is applied to contribute to society now and in the future. Based on this philosophy, the Laboratory of Advanced Materials Chemistry is engaged in revolutionary research. While working on research, Professor Yasuchika Hasegawa actively writes books, appears on TV and attends events for high school students to raise people’s interest in chemistry.
“In the world of chemistry, a variety of materials can be created by combining atoms and molecules. The main aim of applied chemistry is to create technologies and products that are useful for society by utilizing chemistry. In addition to pursuing theorems and truth in chemistry, research is also conducted to serve needs in the real world.”
In the laboratory, photon and electron are manipulated to create and develop cutting-edge nanotechnology materials. Hasegawa’s specialty is photochemistry, and the focus is placed on rare earth elements (Note 1). Some rare earth elements produce luminescence, when they are provided with energy such as ultraviolet light. It is known that europium emits a red light and terbium emits a green light. Professor Hasegawa has developed a material whose luminescence intensity is 100 times larger than inorganic crystals (luminescent material) by synthesizing a complex from rare earth ions and organic molecules.
“With Hasegawa’s First Theory, which I proposed in 1996, I have successfully suppressed thermal oscillation through low-vibration chemical bonding to make neodymium produce intense luminescence. It had been believed that neodymium did not glow in an organic medium. However, I made it by incorporating a new synthesis method based on quantum chemistry (Note 2).”
Furthermore, Professor Hasegawa proposed Hasegawa’s Second Theory in 2003. This is intended to increase the speed of luminescence by making the structure of a complex asymmetric. Rare earth elements are originally less likely to glow. When, however, rare earth complexes with organic ligands make asymmetric structure, d orbitals (even orbitals) are mixed with f orbitals (odd orbitals) of rare earth, resulting in enhancement of luminescence (Note 3). This theory comes from the novel concept of “mixing an electronic orbital”.
“Specific organic matter (phosphine oxide) is attached to core rare earth elements to mix electronic orbitals. Since phosphine oxide promotes not only suppression of vibrational relaxation, but also easy-arrangement of location on a target site. The arrangement seems to make a plastic model. This allowed me to obtain the world’s highest luminous efficiency, and this success has been reported in many scientific journals.”
A luminescent material can also be mixed with plastic and polymer, and can be applied to a variety of industrial products. The development of a material that can freely control light has had a great scholarly and industrial impact on society.
Chameleon luminous body paint whose color changes with temperature
Applicable to improving the efficiency of solar panels
“Hasegawa’s Third Theory is an organic molecule-based solution to the problem of heat-sensitive luminescent complexes. I have developed a complex that can withstand a temperature of up to 300℃ by devising a hydrogen bond network among coordination polymers to strike a balance between intense luminescence and thermal durability. Using this, I have created special paint whose color changes with temperature.”
“This paint named ‘chameleon luminophore’ (Note 4) (Photo 1) shows highly resistant to heat. It produces luminescence even at a high temperature of 200℃, and is not decomposed even at 300℃. Unlike the existing temperature measurement method using electronic sensors (point information), this paint has made it possible to obtain temperature two-dimensional information on a body through color change. It is now applied in wind tunnel tests for supersonic jetliners and rockets as well as endurance tests for chemical reactor vessels.”
A recent study has revealed that the use of the chameleon luminophore can increase the conversion efficiency of solar cells by 2%. A sheet containing rare earth complexes attached on a solar power panel can convert ultraviolet radiation, which cannot be converted by silicon solar cells, to red radiation within the spectral sensitivity range of cells. The durability of cells has also significantly been improved, and there are increasing expectations for practical application.
“The basis of my research is quantum chemistry. Starting from there, I have established three theories that defy common wisdom in materials chemistry. The luminescent material and chameleon luminophore can be applied in diverse fields. I feel increasing expectations from society.”
| Note 1. | Rare earth elements Generic term for a total of 17 chemical elements that include the two elements of scandium (21, Sc) and yttrium (39, Y), as well as 15 elements (lanthanide) from lanthanum (57, La) to lutetium (71, Lu). They are used for magnets, optical disks, lasers, fluorescent substances (e.g., displays, LED), optical fiber amplifiers, condensers, and superconducting materials among other things. The word “rare” is used in the name because it was difficult to chemically classify the elements when they were discovered, but not all the elements are necessarily rare metals. |
|---|---|
| Note 2. | Successful luminescence of Nd (III) in an organic medium by lowering the oscillation of molecules The theory proposed is intended to suppress the vibrational frequency by covering a complex mainly made of neodymium with fluorine atoms. He succeeded in the luminescence of Nd (III) in an organic medium for the first time in the world. |
| Note 3. | Development of the world’s first molecule with an asymmetric nature A molecule mainly made of europium was designed to have an asymmetric structure by setting phosphine oxide in the molecule. Europium emits an intense red light when the outermost orbitals mix. The luminescent efficiency was more than 90%, meaning it displayed the world’s most intense luminescence. |
| Note 4. | Chameleon luminopore When paint made of organic molecules containing terbium and europium atoms is exposed to ultraviolet radiation, it emits yellow composed of green (terbium) and red (europium) lights due to ultraviolet energy. The ratio of energy allocated to atoms varies with temperature, and the luminescent color continuously changes from green to yellow, orange and red. Temperatures from minus 100℃ to 250℃ can be measured from color change. |
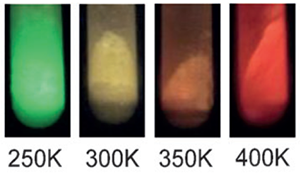
Photo 1. Color change in chameleon luminophore paint

Photo 2. Laboratory
