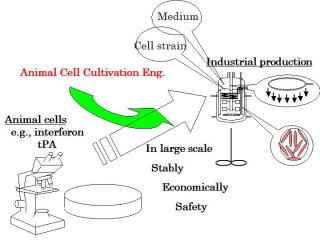
Animal cell cultivation engineering for biologics
Animal cells, unlike single-cell microorganisms, have unique features such as anchorage-dependence, slow growth, low resistance to shear force, and complex nutritional requirements. We have been studying various methods of cultivating animal cells, in order to practice it on a large scale. These include the micro-carrier cultivation method, special dissolved oxygen supply technology, on-line measurement of the oxygen consumption speed, low serum media, controlling protein production using osmotic and static pressure, and the dynamic control of apoptosis.
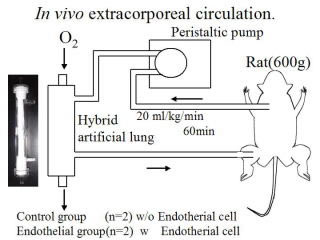
Hybrid artificial lung
In ordinary artificial lungs, gas exchange occurs between the blood and the oxygen through a polypropylene hollow-fiber membrane. However, this artificial material may cause patients to have inflammatory reactions. We have proposed a hybrid artificial lung with endothelial cells adhering to a hollow-fiber membrane, which could lower inflammatory reaction. Various modifications of polypropylene hollow-fiber membrane realized strong adherence of cells to the membrane.
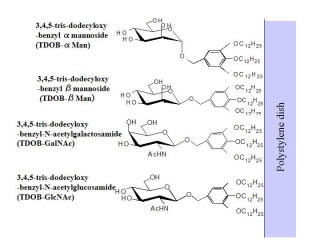
Synthetic glycolipid ligand
Aiming to present cell active ligands including membrane-bound cytokines, extra-cellular matrix, and soluble factors (cytokines, growth factors). Sugar chains, peptide and protein were bound to an artificial lipid and coated on a hydrophobic surface. Activation of hepatic parenchymal cells and amplification of hemopoietic progenitor cells were performed by the presence of galactose and sulfated sugar, repectively.
Ex vivo amplification of hematopoietic progenitor cells
in a three-dimensional co-culture
| Ex vivo amplification of umbilical cord blood hemopoietic progenitor cells is effective in bone marrow transplant medicine. We showed that three-dimensional co-cultivation with hematopoietic cells and stromal cells adhering on a porous carriers could amplify umbilical cord blood hemopoietic progenitor cells without the addition of expensive cytokines. The selection of an apropriate porous carrier for three-dimensional cultivation of stromal cells, and modifications using sulfated sugars, were effective for this amplification. It was suggested that three-dimensional cultivation might contain more amount of insoluble components such as matrix comparing with two-dimensional cultivation on the bottom of a plastic dish. | 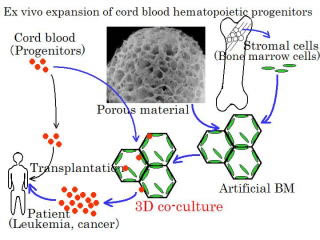 |
Regeneration of cartilage using bone marrow mesenchymal stem cells
| We have developed a convenient method for isolation of mesenchymal stem cells (MSC) from bone marrow without using density gradient centrifugation and safe expansion method of MSC employing donor serum and growth factors instead of fetal calf serum, aiming regeneration medicine of cartilage tissues containing type II collagen and aggrecan. Further, we have improved the efficiency of differentiation of MSC to chondrocytes employing the quantitative RT-PCR method, and proposed a three-dimensional cultivation method of those chondrocyte cells to cartilage tissue. | 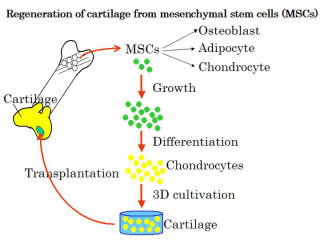 |
Non-invasive estimation of cell quality
| In regenerative medicine, autologous cells were cultivated to create cells and tissues for transplantation. The quality of the cells and tissues produced (especially the status of cell differentiation) must be estimated non-invasively. We have developed a method of estimating the degree of differentiation of mesenchymal stem cells to chondrocytes, based on cell morphology information obtained using microscopic images. We have also developed a method of quantitatively measuring three-dimensional images of cells using a phase shifting laser microscope. | 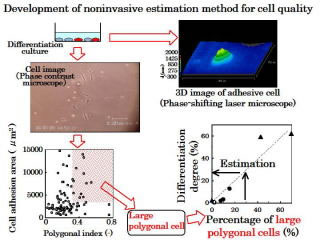 |
