
Long-term evaluation of novel pilot-scale membrane systems for drinking water production
 Researcher:
Researcher:
 Yuichi Tomioka
Yuichi Tomioka
COE Post-Doctoral Fellow
Water Quality Control Eng. Lab.

Introduction
On weathering of arsenic bearing sulfide minerals, arsenic is dissolved and diffused throughout the environment. Arsenopyrite is a very common mineral containing arsenic. There are many studies about the arsenopyrite oxidation mechanism. However, the mechanism to control the valence of arsenic ions extracted from arsenopyrite is not well known. In this study, the factors affecting arsenopyrite dissolution and valence of arsenic ions were investigated.
Mineral Sample
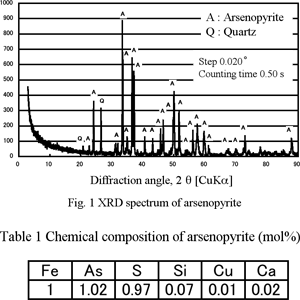
Experimental Leaching Experiments
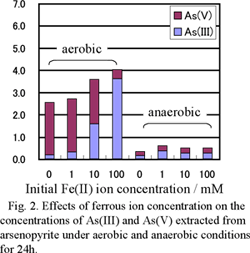
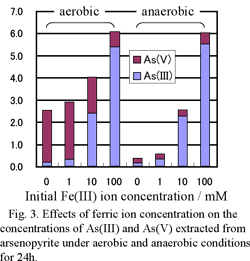
To investigate the effect of Fe(II) and Fe(III), 50 cm3 erlenmeyer flasks were filled with 10 cm3 of 0.1M sulfuric acid containing Fe(II) and Fe(III) (0-100 mM) and 0.1 g of the arsenopyrite. For the experiments under aerobic conditions, the flasks were capped with porous plugs. For the anaerobic experiments, air in the flasks was purged with N2 gas (1 L/min for 3 min) and the flasks were sealed with butyl-rubber plugs. The flasks were shaken in a water bath shaker at 25 ℃ under light shielding conditions. After 24 h, solution was collected by 0.2 μm membrane filter and analyzed to determine the total soluble As (T-As) and As(III) concentration by ICP-AES or the thionalide extraction method 2). The As(V) concentration was calculated by subtracting the As(III) concentration from the T-As concentration.
As(III) Oxidizing Experiment
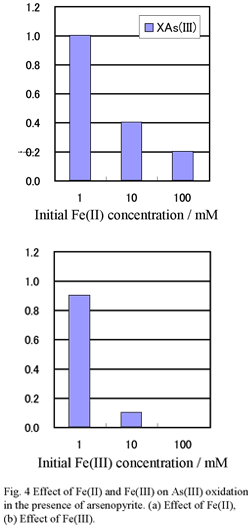
The 50 cm3 of erlenmeyer flasks were filled with 10 cm3 of 0.1 M sulfuric acid containing known concentrations of As(III), Fe(II) and Fe(III), and 0.1 g of the arsenopyrite.
Flasks were capped with porous plugs and shaken for 24 h under aerobic conditions.
Two series of experiments were carried out: in “series A”, 1 mM As(III) was added, and in “series B”, As(III) was not added. In series A, a part of the added As(III) is oxidized after 24 h. The amount of the oxidized As(III), XAs(III), is estimated by the following equation.
XAs(III) = [As(III)]d + [As(III)]0h - [As(III)]24h
[As(III)]d: As(III) concentration at 24h in the experiments in series B.
[As(III)]0h: Initial As(III) concentration at 24h in the experiments in series A
[As(III)]24h:As(III) concentration at 24h in the experiments in series A
3. RESULTS AND DISCUSSIONS
Conclusions
Concentrations of Fe(II) or Fe(III) are an important factor in determining the valence of arsenic ion extracted from arsenopyrite under acidic conditions. The As(III) is oxidized to As(V) in the presence of arsenopyrite, but high concentrations of Fe(II) or Fe(III) suppress the oxidation, and reduce the As(V) concentration.
References
1) K. Sasaki, M. Tsunekawa, S. Tanaka, M. Fukushima, H. Konno: Shigen-to-Sozai, 115(1999), 233-239
2) S. Nakaya: Bunseki Kagaku, 12(1963), 241-24.
