
Long-term evaluation of novel pilot-scale membrane systems for drinking water production
| Tel: (011) 706-6267 |
 Researcher:
Researcher:
 Soryong Chae
Soryong Chae
Water Quality Control Eng. Lab.

ABSTRACT:
Novel pilot-scale microfiltration membrane (polyvinylidene fluoride, PVDF) having different pore size (0.1 and 0.05μm) filtration systems including pre-treatment that are consisted of chemical coagulation, sedimentation by a jet mixed separator (JMS), and a rapid sand filter (RSF) in series have been evaluated for two months. As a result, turbidity was removed completely and organic matter, humic substances, and metals (Al, Fe) were removed very well by the proposed system. In addition, it was concluded that a membrane having dense layer on the skin was more effective to reduce membrane fouling than a membrane having homogeneous structure although the nominal pore size of the former (0.05μm) was smaller than that of the latter (0.1μm).
Keywords: Pre-treatment; microfiltration; PVDF; membrane fouling.
1. INTRODUCTION
Membrane filtration using microfiltration (MF) and ultrafiltration (UF) membranes has been widely used to produce drinking water from river, lake and underground water (Ma et al., 1998; Yuasa, 1998; Klijn et al., 2000; Kimura et al., 2004). These low-pressure membranes can remove particulate and colloidal matters bigger than the pore size of the membrane and the filtrate is usually free of turbidity and bacteria (Hagen, 1998). However, membrane fouling that was mainly caused by natural organic matter (NOM), suspended particles, and inorganic matters limits the long-term operation of the membranes (Fane et al., 1987; Kaiya et al., 1996). To overcome this defect, combination with other processes such as coagulation/sedimentation and activated carbon has been adopted (Yuasa, 1998; Jang et al., 2001).
In this study, two kinds of pilot-scale novel membrane filtration systems for drinking water production have been fabricated and operated to challenge current problems related both to the effective removal of pollutants from water and the reduction of membrane fouling. The performance of the membrane filtration depends on the raw water quality and the operating conditions such as filtration flux, pressure, backwash method, etc. Therefore, the feasibility of the new membrane processes should be evaluated by a long-term pilot test applied to the actual raw water.
2. MATERIALS AND METHODS
For this study, Chitose River water in the Kami Ebetsu Water Purification Plant (Ebetsu City, Japan) has been used as raw water. Although water quality of Chitose River changed seasonally, especially it contains relatively high turbidity, organic matter, and humic substances in summer and fall (Bian et al., 1999; Jang et al., 2001).
2.2 Experimental set-up
The membrane filtration system is composed of coagulation/sedimentation by a jet mixed separator (JMS, Watanabe et al., 1998), a rapid sand filter (RSF) and two membranes having different structure and pore size. Details of the composition of this system are summarized in Table 1.
Turbidity was analyzed using an integration ball type turbidity meter (SEP-PT-706D, Mistubishi Chemical Co., Japan) after ultra-sonification for 10 seconds. To check temperature and pH, a portable pH meter (HM-12P, TOA electronics Ltd., Japan) was used. Humic substances (UV260) was measured by Hitachi spectrophotometer (Model U-2000, Japan) with a one cm cell at 260 nm, and total organic carbon (TOC) and dissolved organic carbon (DOC) were measure by a Shimadzu TOC meter (Model TOC-5000, Japan). Inorganic matters such as Al, Mn, Fe were determined by an inductively coupled plasma atomic emission spectrophotometer (ICPS-7500, Shimadzu Co., Japan).
3. RESULTS AND DISCUSSIONS
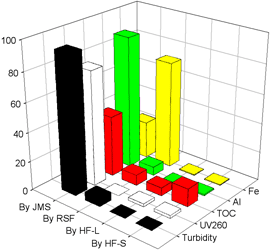
Fig. 1 Removal efficiencies of various
pollutants in the membrane system.
As shown in Fig. 3, most of turbidity (94.1%), humic substances (78.5%), TOC (41.8%), and total aluminum (90.4%) were removed by the JMS with pre-coagulation. However, total iron (73.2%) that was mostly supplied as coagulant was rejected by the RSF. In term of TOC, the removal by the HF-S (0.05μm) was about 7% higher than by the HF-L (0.1μm).
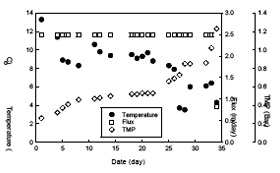
Fig. 2 Variations of flux and TMP in the HF-L
membrane (0.1μm).
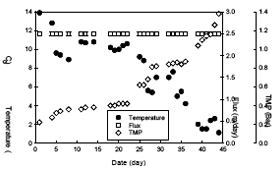
Fig. 3 Variations of flux and TMP in the HF-S
membrane (0.05μm).
Fig. 2 and 3 show that variations of temperature, permeate flux and TMP of the HF-L and the HF-S membrane, respectively. As shown in those figures, in the intial step, the TMP was maintained at about 0.2 Bar until 5 days and then increased gradually as temperature incresed. Finally, it took about 35 and 45 days to reach the TMP of 1.2 Bar in the the HF-L and the HF-S membrane, respectively. Therefore, from this study, it was concluded that the HF-S membrane having dense layer on the skin was more effective to reduce membrane fouling than the HF-L membrane having homogeneous structure although the nominal pore size of the HF-S was smaller than that of the HF-L.
4. CONCLUSIONS
In this study, two PVDF membranes having different pore size and structure was newly operated with pre-treatment to produce portable water from the surface water. As a result, turbidity was removed completely and organic matter, humic substances, and metals (Al, Fe) were removed very well by the proposed system. In addition, it was concluded that a membrane having dense layer on the skin was more effective to reduce membrane fouling than a membrane having homogeneous structure although the nominal pore size of the former (0.05μm) was half of the latter (0.1μm)
For the second opearation mode, only the coagulant was changed from PSI to PAC (polyaluminium chloride) under the same condition. Performance study of the membrane filtration system and modeling about effects of temperature and water quality on membrane fouling will be conducted.
References
Bian, R., Watanabe, Y., Tambo, N. and Ozawa, G. (1999) Removal of humic substances by UF and NF membrane systems. Wat. Sci. Tech., 40(9), 121-129.
Fane, A. G. and Fell, C. J. D. (1987) A review of fouling and fouling control in ultrafilration. Desalination, 62, 117-136.
Hagen, K. (1998) Removal of particles, bacteria and parasites with ultrafiltration for drinking water treatment. Desalination, 119, 85-91.
Hasegawa, T., Hasimoto, K. and Tambo, N. (1991) Characteristics of metal-polysilicate coagulants. Wat. Sci. Tech., 23, 1713-1722.
Jang, N-Y, Watanabe, Y., Minegishi, S. and Bian R. (2001) The evaluation of dead-end ultrafiltration membrane process combined with pre-coagulation/sedimentation. JWWA, 70(2), 1-14.
Kaiya, Y., Itoh, Y., Fujita, K., Takizawa, S. (1996) Study on fouling materials in the membrane treatment process for portable water. Desalination, 106, 71-77.
Klijn, R. B., van der Meer, W. G. J., Vriezen, H., van Ekkendonk, F. H. J. (2000) Surface water treatment with Zenon microfiltration membranes: minimisation of energy and chemical use. Desalination, 131, 337-343.
Kimura, K., Hane, Y., Watanabe Y., Amy, G., Ohkuma, N. (2004) Irreversible membrane fouling during ultrafiltration of surface water. Wat. Res., 38, 3431-3441.
Ma, W., Sun, Z., Wang, Z., Feng, Y. B., Wang, T. C., Chan, U. S., Miu, C. H., Zhu, S. (1998) Application of membrane technology for drinking water. Desalination, 119, 127-131
Watanabe, Y., Kasahara, S. and Iwasaki, Y. (1998) Enhanced flocculation/sedimentation process by a jet mixed separator. Wat. Sci. Tech., 37(10), 55-67.
Yuasa, A. (1998) Drinking water production by coagulation - microfiltration and adsorption - ultrafiltration. Wat. Sci. Tech., 37(10), 135-146.
